Question
Question: The increasing order of basicity of the above compounds is: (a)  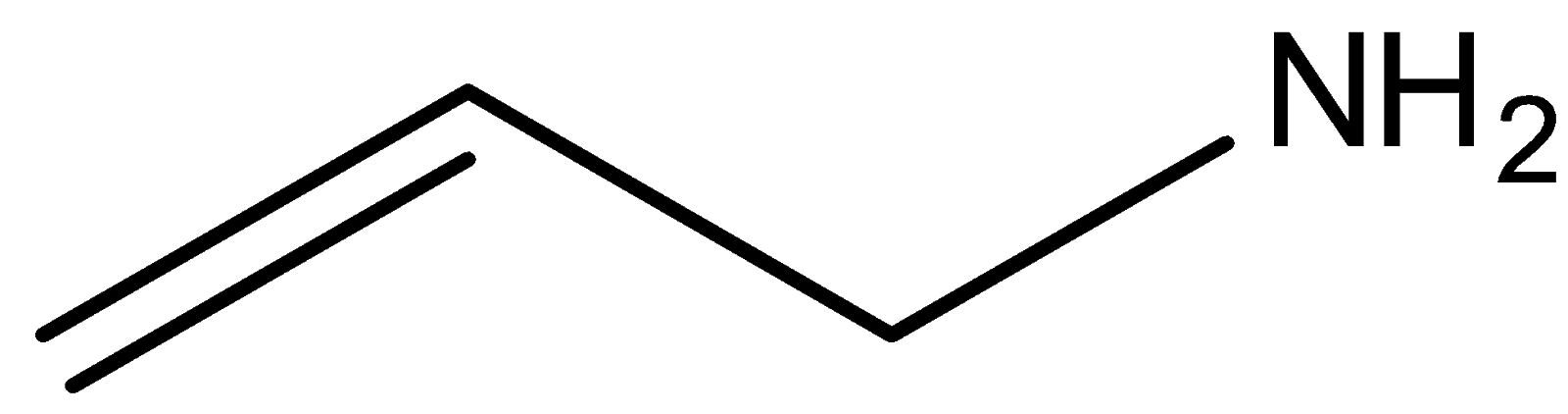
(b)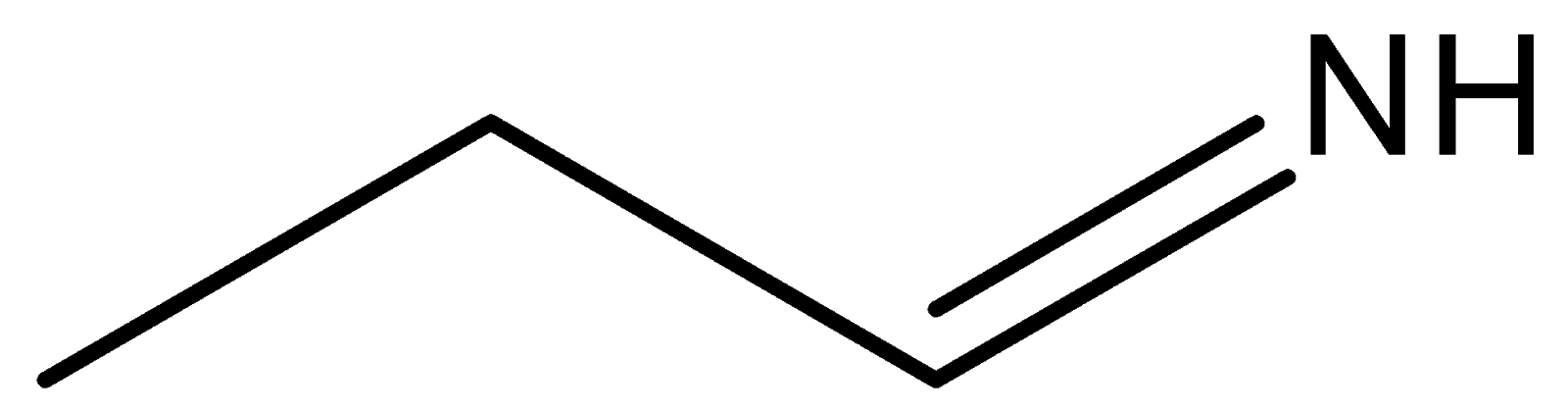
(c) 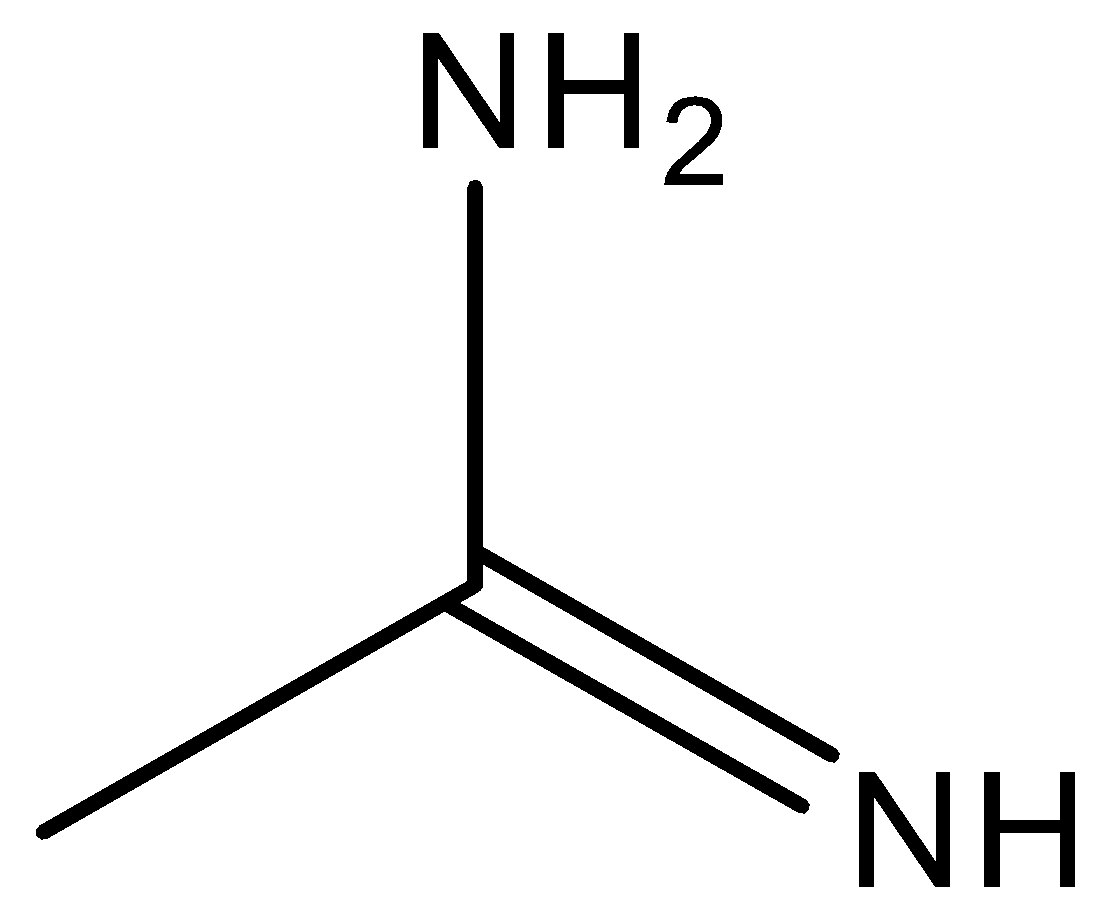
(d) 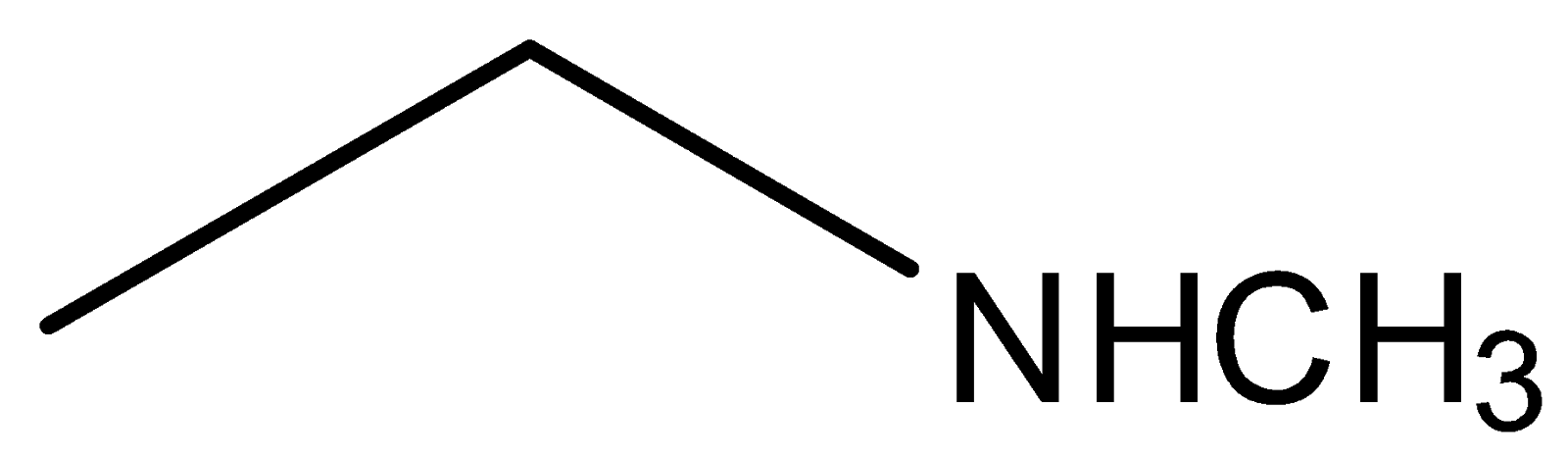
A.b < a < d < c
B.d < b < a < c
C.a < b < c < d
D.b < a < c < d
Solution
Basicity of a compound depends on the ability of the compound to donate electrons. The donating ability of a compound also depends on the hybridization. It decreases as the lone pair of electrons get involved in resonance and become unavailable for donation.
Complete step by step solution:
In compound (b), the nitrogen atom is sp2 hybridised, so its ability to donate electrons will be less due to greater s character, which pulls the electron towards the nucleus. Compounds (a) and (d) are primary and secondary amines. Out of these two compounds, the compound (d) is stronger compared to compound (a) because of the +Ieffect of the methyl group. Compound (a) is a weaker base than compound (d) because of the −I effect caused by the group present on compound (a).
Compound (c) is considered to be a very strong nitrogenous base because of its ability to create a negative charge on one of the nitrogen atoms while the other nitrogen atom delocalised its lone pair of electrons over the bond. It creates a partial double bond character to the bond. So, compound (c) is the strongest base.
The basicity of compounds can be predicted by looking at the conjugate acid produced by the base and its stability also. The stronger the base, the weaker will be its conjugate acid and vice versa.
Hence, the option (A) b < a < d < c is the correct option.
Note:
Remember that the basic strength of a compound always depends on the hybridization, electron donation ability and the strength of conjugate acid or conjugate base formed by the compound. It also depends on the stability of conjugate acid or base formed.
