Question
Question: The increasing order of basicity for the following intermediates is (from weak to strong) 
(A) (v)<(i)<(iv)<(ii)<(iii)
(B) (v)<(iii)<(ii)<(iv)<(i)
(C) (iii)<(iv)<(ii)<(i)<(v)
(D) (iii)<(i)<(ii)<(iv)<(v)
Solution
Basicity in organic chemistry is determined by the degree of acceptance of protons by a particular molecule. Out of all the given options above, the most basic molecule would be the one which is least stable.
Complete step-by-step answer:
A molecule is more basic if its readiness to accept protons or donate electrons is relatively more than its other counterparts. As you already know, acids and bases are defined as proton donors and proton acceptors in the Bronsted-Lowry concept respectively; whereas they are defined as electron acceptors and electron donors in the Lewis concept respectively.
So, to find out the molecules which are the most and least basic from the given options, we have to determine the willingness of each molecule to accept a proton by donating an electron. That means, the molecule which is the most unstable in its present configuration is more basic than others because it will readily accept a proton so that it becomes stable. Going along this concept, we can state the following points about the individual molecules present in the option:
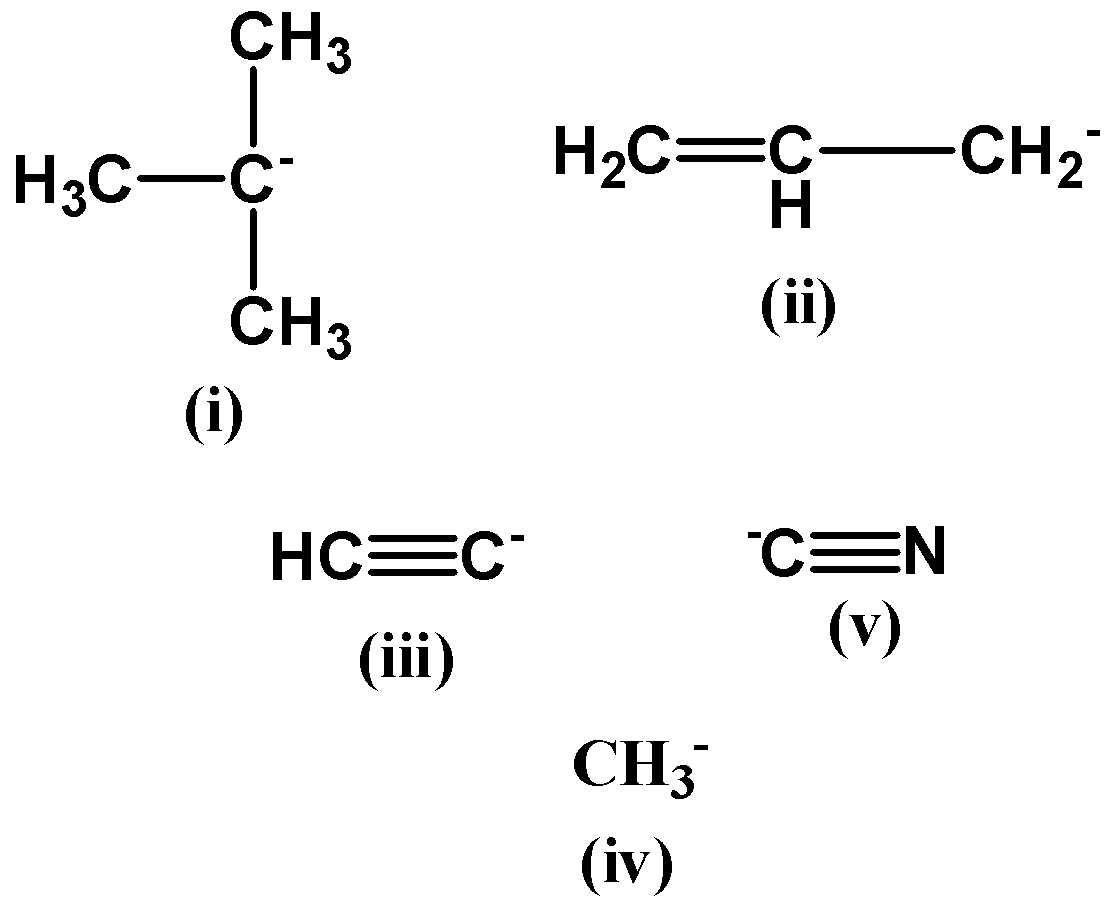
The negative charge is present on the tertiary carbon atom. Due to the collective inductive effect of all the methyl groups attached to it, the tertiary carbon is already electron rich and therefore the negative charge actually destabilizes its overall structure.
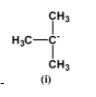
The negative charge is situated on the allylic carbon. Due to resonance this negative charge is distributed on the overall molecule making it relatively stable. But the fact that this negative charge is carried by carbon atoms which are not electronegative in nature makes it unstable than those atoms which are inherently electronegative such as nitrogen.
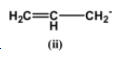
The carbons here are sp hybridized due to the presence of triple bonds. This makes them more electronegative than all the hybridised forms of carbon such as sp2or sp3.
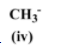
The methyl anion is more stable than the tertiary carbon (first option) because it does not have electron donating groups attached to it which otherwise would have destabilized the negative charge on it.
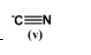
This anion is the most stable because it has a carbon which is sp hybridized along with an electronegative atom which is nitrogen.
So, taking into account our above observations, the increasing order of stability is-
(i)<(iv)<(ii)<(iii)<(v)
And therefore the increasing order of basicity of these molecules is just the opposite of the above one, which is option (B)- (v) <(iii)<(ii)<(iv)<(i)
Note: Some students might face confusion regarding the order between the molecules in option (ii) and (iii). Always remember that the more stable molecule will have relatively more electronegative atoms. In this case, option (iii) is more stable because it has sp hybridized carbon atoms.
