Question
Question: The incorrect geometry is represented by: A) \[{\text{N}}{{\text{F}}_3}\] - trigonal planar B) \...
The incorrect geometry is represented by:
A) NF3 - trigonal planar
B) H2O - bent
C) AsF3 - Trigonal bipyramidal
D) BF3 - trigonal planar
Solution
To determine shapes or geometry of molecules we need to take into account the lone pair and bond pair, On the basis of various combinations geometry and shape of molecule changes.
Complete step by step solution:
The central atom in NF3 is nitrogen, it has five valence shell electrons. Out of five valence shell electrons 3 electrons are forming bonds with fluorine and are known as bond pairs and 2 electrons exist as lone pairs. The geometry formed out of 3 bond pairs and 1 lone pair is trigonal pyramidal and not trigonal planar. Hence it is an incorrect representation of geometry.
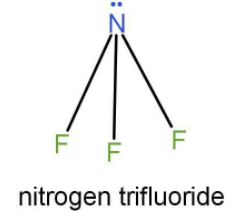
Hence, the correct option is A.
Additional information:
In H2O, the central atom is oxygen which has 6 valence electrons. Out of the six valence electrons 2 electrons are forming bonds with hydrogen and are known as bond pairs and 4 electrons exist as 2 lone pairs. The geometry formed out of 2 bond pairs and 4 lone pairs is tetrahedral and the shape becomes bent.
The central atom in AsF3 is arsenic; it has five valence shell electrons. Out of five valence shell electrons 3 electrons are forming bonds with fluorine and are known as bond pairs and 2 electrons exist as lone pairs. The geometry formed out of 3 bond pairs and 1 lone pair is pyramidal.
In BF3, the central atom is boron which has 3 valence electrons. All the three electrons are bond pairs. Hence the geometry and shape will be trigonal planar.
Note:
Generally geometry and shapes are considered the same. In geometry we account for the bond pair and lone pair as well but in case of shape we do not consider lone pair, we only consider bond pairs. For example the shape of NF3 is pyramidal but the geometry of NF3 is tetrahedral.
