Question
Question: The hybridization state of the central atom in \({\rm{PC}}{{\rm{l}}_{\rm{5}}}\) is: A. \(s{p^3}d\...
The hybridization state of the central atom in PCl5 is:
A. sp3d
B. sp3d2
C. sp3
D. d2sp3
Solution
Phosphorus pentachloride is the commonly abbreviated as PCl5. PCl5 has a trigonal bipyramidal structure.
Complete step by step answer:
We know that, in phosphorus pentachloride, the phosphorus is the central atom. The electronic configurations of phosphorus and chloride atoms is as follows:
Phosphorous- (1s22s22p63s23p3) and chloride- (1s22s22p63s23p5)
So, one of the “s”, three of the “p” and of the “d” orbitals participate to give a 3pz hybridisation. The five orbitals of the sp3d hybrid is occupies singly, so it overlaps with the 3pz orbital of the chlorine atom forming five sigma P-Cl bonds. This gives rise to the trigonal bipyramidal shape of the PCl5 molecule having the bond angles of 90∘ and 120∘.
We can draw the structure of PCl5 as follows:
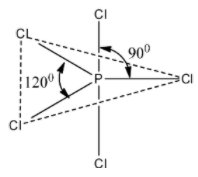
The above PCl5 structure that we have drawn is a trigonal bipyramidal structure. Due to the trigonal bipyramidal structure, the two Cl− atoms of PCl5 molecule are stretched and lie along the axis and it becomes little longer and so these bonds called as axial bonds, while the other three Cl− atoms lie on equator and these bonds are called as equatorial bonds.
So, out of the given four options, A is the correct option.
Note:
Students may get confused in interpreting the structure of the PCl5. It should be noted that in PCl5, two P−Cl bonds remains as axial bonds along the axis and the other three P−Cl bonds remains as equatorial bonds.
