Question
Question: The hybridization state of beryllium atoms in \(BeC{l_2}\) a molecule is x. The type of hybridizatio...
The hybridization state of beryllium atoms in BeCl2 a molecule is x. The type of hybridization changes to y when BeCl2 transforms to the solid-state. The x and y are respectively:
A.sp,sp2
B.sp,sp3
C.sp2,sp3
D.sp,sp
Solution
Hybridization is defined as the concept of intermixing of the orbitals of an atom having nearly the same energy to give exactly equivalent orbitals with the same energy, identical shapes, and symmetrical orientation in space.
Complete step by step answer:
Hybridization of any element can be found by using the formula:
X=21[V+M−C+A]
Where V = number of valence electrons of the central metal atom.
M = number of monovalent groups.
C = number of positive charges.
A = number of negative charges.
We would utilise the same formula to calculate the hybridization of BeCl2. Here the central atom is beryllium and since the atomic number of Beryllium is 4 and it belongs to group second hence the number of valence electrons is 2. Since chlorine is a monovalent atom hence, in case of BeCl2, there are 2 monovalent atoms. And there are no overall charges hence hybridization will be:
X=21[2+2]
⇒X=21×4=2
Hence the hybridization is sp and the structure is linear at nearly 1200k.
The structure will be:

But in the case of the solid phase, BeCl2 has a polymeric chain structure. The polymeric structure of the BeCl2 is due to its electron deficiency. Beryllium has only four-electrons in the valence shell hence, it can accept two more electron pairs from neighbouring chlorine chains forming coordination bonds. In this case hybridization of beryllium in BeCl2 getting changes to sp3. The polymeric chain structure BeCl2 is given as: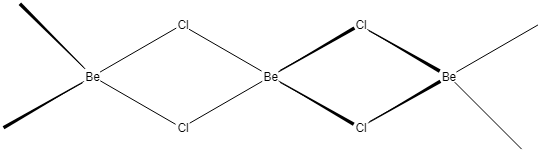
Hence the x and y are respectively sp and sp3.
Hence the correct answer is option is B.
Note:
Beryllium chloride acts as a strong lewis base due to its electron-deficient nature and is used as a catalyst in the Friedel-craft reaction. It is a covalent compound hence, it is soluble in the organic solvent.
