Question
Question: The hybridization of the central atom \(S\) in \(S{{O}_{2}}\) is: A. \(s{{p}^{2}}\) B. \(s{{p}^...
The hybridization of the central atom S in SO2 is:
A. sp2
B. sp3
C. spd3
D. d3s
Solution
We can calculate the steric factor for this molecule and then according to this number, we can assign the hybridization to this molecule. Steric factor of any molecule is the sum of the sigma bonds it contains and the number of its lone pairs.
Complete answer:
First, let us see the definition of hybridization.
- Hybridization is the phenomenon of mixing of orbitals of slightly different energies so as to redistribute their energies resulting in the formation of a new set of orbitals of equivalent energies and shape called degenerate orbitals.
- To predict the hybridization of a given molecule, first calculate the steric number (X) of the given molecule. The steric Number (X) is equal to the sum of the number of sigma bonds around that atom and the number of lone pairs on that atom. This can be represented as:
Steric factor (X ) = number of sigma bonds + number of lone pairs.
- The structure of SO2 is:
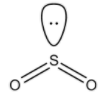
- It is known to you that hybridization for the given steric values is as follows:
