Question
Question: The hybridization of phosphorus in \(POC{l_3}\) is the same as in: A.P in \(PC{l_3}\) B.S in \(...
The hybridization of phosphorus in POCl3 is the same as in:
A.P in PCl3
B.S in SF4
C.Cl in ClF3
D.B in BCl3
Solution
Hybridization is defined as the intermixing of orbitals to form new hybrid orbitals. It is basically a concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics.
Complete step by step solution:
During the process of hybridization, the atomic orbitals of similar energy are mixed together to form new hybrid orbitals. It happens only during the bond formation and not in an isolated gaseous atom. Moreover, the shape of the molecule can be predicted if the hybridization of the molecule is known. There are different types of hybridization such as sp3,sp2,sp3d2,sp3d3 and many more.
Now, in case of POCl3 and in case of PCl3, P atom is sp3 hybridized. In PCl3, P atom has three bond pair of electrons and one lone pair of electrons whereas in case of POCl3, P atom has for bonding domains which include one P=O double bond. Both the structures are as shown:
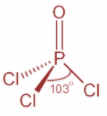
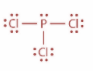
Now, when one ‘s’ orbital and three ‘p’ orbitals belong to the same shell of an atom mix together to form four new equivalent orbitals then this type of hybridization is known as tetrahedral hybridization or sp3 hybridization. Example methane.
Hence, option A is correct.
Note:
Don’t get confused with molecular and hybrid orbitals. The interaction between the atomic orbitals of two different atoms result in molecular orbitals whereas when atomic orbitals of the same atom interact, then they form hybrid orbitals.
