Question
Question: The hybridization of \[PC{{l}_{5}}\] molecule is: (a)- \[s{{p}^{3}}\] (b)- \[s{{p}^{2}}{{d}^{2}}...
The hybridization of PCl5 molecule is:
(a)- sp3
(b)- sp2d2
(c)- sp3d
(d)- sp2
Solution
Hint : When one s, three p and one d-atomic orbitals mix, it forms five sp3d hybrid orbitals of equal energy called sp3d hybridization.
When one s, three p and two d orbitals mix, it forms six identical hybrid orbitals called sp3d2 hybridization.
Similarly, on mixing one s and three p orbitals, three identical hybrid orbitals are formed called sp3 hybridization.
Complete step by step solution :
The electronic configuration of phosphorus and chlorine is [Ne]3s23p3 and [Ne]3s23p5respectively. We can see that the number of valence electrons in the phosphorus atom is 5, here in PCl5, according to VSEPR theory it will form a single bond with each of the chlorine atoms i.e. Phosphorus atom needs five orbitals to form the five P-Cl bonds.
It has two electrons in its 3s orbital and three in 3p orbitals in the valence shell, so it must use one of its 3d to form the fifth bond, So, one electron from 3s gets excited to the d orbital and they form five hybrid orbitals.
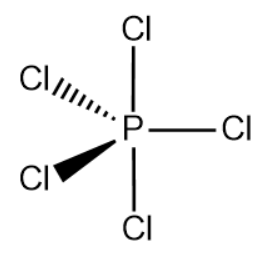
Therefore, the hybridization of PCl5 id sp3d.Hence, the correct option is (c).
Additional Information:
Phosphorus pentachloride is a greenish-yellow solid, having a trigonal bipyramid structure. It is readily soluble in water.
Note : Hybridization of a molecule can also be calculated by a formula:
Hybridization = 21(V+M−C+A)
where, V = valence electrons,
M = monovalent atom linked to central atom,
C = charge on cation,
A = charge on anion.
After getting the numerical value,
2 = sp
3 = sp2
4 = sp3
5 = sp3d
6 = sp3d2
