Question
Question: The hybridization of \(NO_{3}^{-}\) is the same as the hybridization of _________. (A) C in \(C{{H...
The hybridization of NO3− is the same as the hybridization of _________.
(A) C in CH4
(B) C in C2H2
(C) C in C2H4
(D) C in C3H8
Solution
Draw the structure of NO3− and find out its hybridization. In the options, some hydrocarbons are given. We know the kind of hybridization in alkanes, alkenes and alkynes. Match the corresponding hybridization to get the answer.
Complete answer:
- Let’s start by writing the electronic configuration of nitrogen and oxygen.
7N=1s22s22p3
8O=1s22s22p4
- The number of valence electrons in nitrogen is 5 and in oxygen is 6. In nitrate, NO3−, there is one nitrogen and three oxygen atoms and a -1 charge.
- Therefore, the total number of electrons present in nitrate is 5+(3×6)+1=24
- Therefore, the total number of electrons present in nitrate is 224=12
- So, the structure of nitrate is
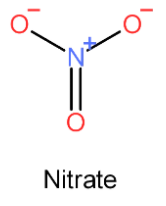
- From the structure, we can conclude that nitrogen forms three !!σ!! -bonds and one !!π!! -bond.
- Therefore, nitrate is sp2 hybridized due to the presence of one double bond which indicates lateral overlapping of 2p orbitals of nitrogen and oxygen.
- Now, let’s have a look at the options.
(A) C in CH4- In methane, carbon is sp3 hybridized.
(B) C in C2H2- In acetylene, carbon is sp hybridized.
(C) C in C2H4- In ethene, carbon is sp2hybridized.
(D) C in C3H8- In propane, carbon is sp3 hybridized.
- Therefore, the hybridization of NO3− is same as the hybridization of C in ethene, C2H2.
Therefore, option (C) is the correct answer.
Note:
Remember the presence of double bond indicates lateral overlapping of unhybridized orbitals. Also, for such problems, derive the structure of the compound or molecule or ion first and then go for hybridization.
