Question
Question: The hybridization of \({[NiC{l_4}]^{2 - }}\) and \({[Ni{(CN)_4}]^{2 - }}\) considering hybridization...
The hybridization of [NiCl4]2− and [Ni(CN)4]2− considering hybridization of the metal ion are respectively.
A.sp3,dsp2
B.dsp2,sp3
C.Both sp3
D.Both dsp2
Solution
Hybridization is defined as the concept of intermixing of the orbitals of an atom having nearly the same energy to give exactly equivalent orbitals with the same energy, identical shapes, and symmetrical orientation in space.
Complete step by step answer:
In the case of the coordination compound, we will find the hybridization with the help of valence bond theory. In the case of [NiCl4]2−, first we find the oxidation state of the central metal atom i.e. Ni. Let us consider the oxidation state of Ni is x. Since chlorine is halogen, hence the oxidation state of chlorine is -1. There for the oxidation state of Ni is calculated as below:
x+4×(−1)=−2
⇒x−4=−2
⇒x=−2+4=2
Hence the oxidation state of Niis +2 and since the atomic number Ni is 28. Its outer electronic configuration is 3d84s2 and it is represented as below:
Ni in ground state:
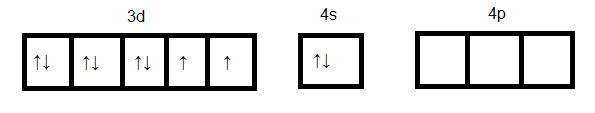
Ni in +2 oxidation state:
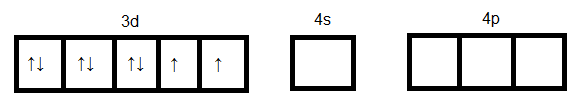
Formation of [NiCl4]2−
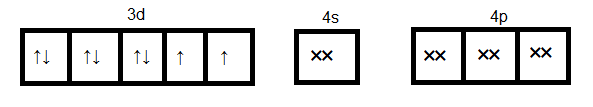
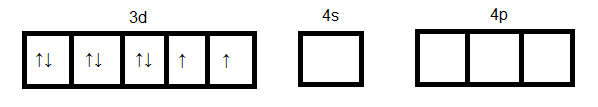
As we see in the above case d orbital cannot participate in hybridization. Four sp3 orbitals can be formed by mixing one 4s and three 4p orbitals. So four hybrid orbitals accommodate four pairs of electrons from four chloride ions. Hence the hybridization will be sp3 and geometry will be tetrahedral
In the case of [Ni(CN)4]2−, we will again find the oxidation state of nickel. Suppose the oxidation state of nickel is x and the charge present on cyanide ion is a negative one and the overall charge present is negative two. Hence, the value of x can be calculated as:
x+4(−1)=−2
⇒x=−2+4=2
Hence the oxidation state of nickel is +2 and its state is represented below
Ni in ground state: 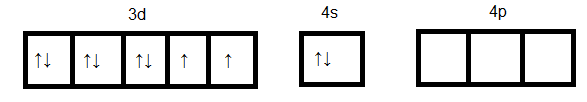
Ni in +2 oxidation state:
Since cyanide is a strong ligand hence pairing of electrons occurs. Formation of [Ni(CN)4]2−
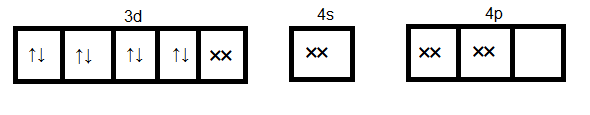
Here dsp2 orbitals accommodate four pairs of the electron from four cyanide ions. Hence the hybridization is dsp2 and geometry will be a square planner
Hence the correct answer is option is A.
Note:
Here [NiCl4]2− is paramagnetic due to the presence of an unpaired electron whereas in case of [Ni(CN)4]2− due to the presence of a paired electron it is diamagnetic.
