Question
Question: The hybridization of \( Ni \) in \( Ni{(CO)_4} \) is: A. \( s{p^2} \) B. \( ds{p^2} \) C. \(...
The hybridization of Ni in Ni(CO)4 is:
A. sp2
B. dsp2
C. sp3
D. sp3d
Solution
Hint : It is a coordination number 4 complex and the complexes having coordination number 4 have two geometry that is tetrahedral and square planar. Tetrahedral geometry is shown when the hybridization is sp3,d3s and square planar geometry is shown when the hybridization is dsp2,sp2d .
Complete Step By Step Answer:
Coordination number is the total number of atoms or molecules bonded to the central atom. Coordination number is also called ligancy which means number of ligands bonded to the central metal atom.
In Ni(CO)4 , Ni is attached to 4 molecules of CO that’s why its coordination number is 4 .
In coordination chemistry, hybridization depends on the number of d-electrons (of the central metal atom). In case of Ni(CO)4 the central metal atom is nickel so we have to count the number of d-electrons in nickel:
Configuration of nickel is as follows:
Ni = 1s22s22p63s23p63d84s2
Valence shell configuration: 3d84s2

As nickel has zero charge on because carbonyl is a neutral ligand now, as we can see that s is not vacant so 4 ligands can't get bonded to nickel.
For bonding in such cases, electrons of s orbital get shifted to d orbital and new configuration becomes 3d104s0 .
It is called d−s pairing and the d10 is called a converted d′system .
3d104s04p0 :
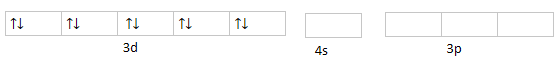 Hybridization will take place with s and p orbital as sp3 . Hence, nickel is sp3 hybridized with geometry tetrahedral and spin only moment zero. Spin only moment is zero because the number of unpaired electrons are zero. As one of its properties is also derived by the number of unpaired electrons that is magnetism. There are no unpaired electrons so it is a diamagnetic compound.
Hybridization will take place with s and p orbital as sp3 . Hence, nickel is sp3 hybridized with geometry tetrahedral and spin only moment zero. Spin only moment is zero because the number of unpaired electrons are zero. As one of its properties is also derived by the number of unpaired electrons that is magnetism. There are no unpaired electrons so it is a diamagnetic compound.
So, option C is correct.
Note :
Ni(CO)4 was first synthesised in 1890 by Ludwig Mond by the direct reaction of nickel metal with CO . This pioneering work foreshadowed the existence of many other metal carbonyl compounds, including those of V,Cr,Mn,Fe and Co . It was also applied industrially to the purification of nickel by the end of the nineteenth century.
