Question
Question: The hybridization of iron in \({K_4}[Fe{(CN)_6}]\) is? (A) \(ds{p^2}\) (B) \(s{p^3}\) (C) \(...
The hybridization of iron in K4[Fe(CN)6] is?
(A) dsp2
(B) sp3
(C) d2sp3
(D) d2sp2
Solution
First find out the oxidation state of iron in the complex and write its electronic configuration. Arrange the atoms keeping in mind that it is attached to a strong field ligand. Now see which orbitals will be involved in bonding with the ligand and accordingly see the hybridisation.
Complete step by step answer:
-First of all we will find out the oxidation state of Fe in the complex K4[Fe(CN)6].
For any complex compound its overall charge is always equal to the sum of the oxidation states of all the constituent atoms and ligands.
The overall charge on the given complex is = 0
Oxidation state of ligand (CN) is = (-1)
Oxidation state of K here = (+1)
Let the oxidation state of Fe here be = x
So, O.S. of complex = 4(O.S. of K) + O.S. of Fe + 6(O.S. state of CN)
0 = 4 (+1) + x + 6 (-1)
0 = 4 + x – 6
x = +2
Here the oxidation state of iron is +2 .
-We know that atomic number of Fe is 26 and hence its electronic configuration in neutral state will be: [Ar]3d64s2
When iron exists as Fe+2 it has a configuration of: [Ar]3d6 and the orbitals split into eg and t2g orbitals with eg at higher energy than t2g.
-In the complex we can see that iron has a coordination number of 6 and the ligand attached to it which is CN− is a strong field ligand and thus causes pairing of the electrons in the d subshell and so the 6 electrons from the 3d subshell accommodate in just the 3 t2g orbitals as shown below:
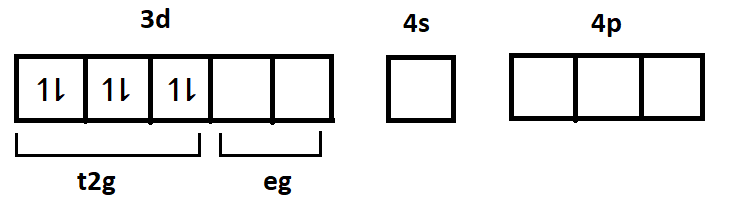
The remaining 2 orbitals from d subshell (e.g. orbitals) will participate in hybridisation. Since the coordination number of iron here is 6 and the ligand is cyanide ion (CN−), the 6 cyanide ions will donate their electron pair and get attached with the 2 ‘3d’ orbitals, 1 ‘4s’ orbital and 3 ‘4p’ orbitals ahead to form the complex. Hence the hybridisation of iron in this complex will be d2sp3.
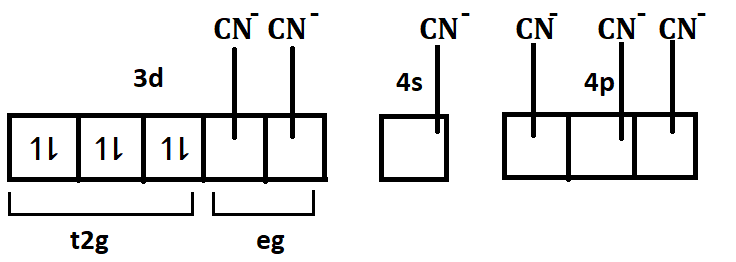
The correct option will be: (C) d2sp3
Note: Ligands which cause a large splitting are known as strong field ligands (like CO, CN−, NO2−, etc), while those which cause small splitting are known as weak field ligands (like Cl−, Br−, F−, etc). Due to the large splitting energy, the strong field ligands cause pairing of the electrons, since large energy input will be required for the electrons to pass this energy barrier.
