Question
Question: The hybridization of iodine in iodosobenzene: (a)- \(sp\) (b)- \(s{{p}^{2}}\) (c)- \(s{{p}^{...
The hybridization of iodine in iodosobenzene:
(a)- sp
(b)- sp2
(c)- sp3
(d)- sp3d
Solution
If the atom has a single bond, the hybridization is sp3. If the atom has a double bond then the hybridization is sp2. If the atom has a triple bond then the hybridization is sp.
Complete step by step answer:
-All the orbitals in an atom are not of the same energy, so when the atoms attach with other atoms then these energies redistribute among themselves and become equal in energy, and this process is known as hybridization. With the help of hybridization, we can predict the number of orbitals in the atom of the molecule. For finding the hybridization of the carbon atom, the rules are as follows:
-If the iodine atom has a single bond, the hybridization is sp3.
-If the iodine atom has a double bond then the hybridization is sp2.
-If the iodine atom has a triple bond then the hybridization is sp.
-So the molecule iodosobenzene has a formula C6H5IO and its structure is given below:
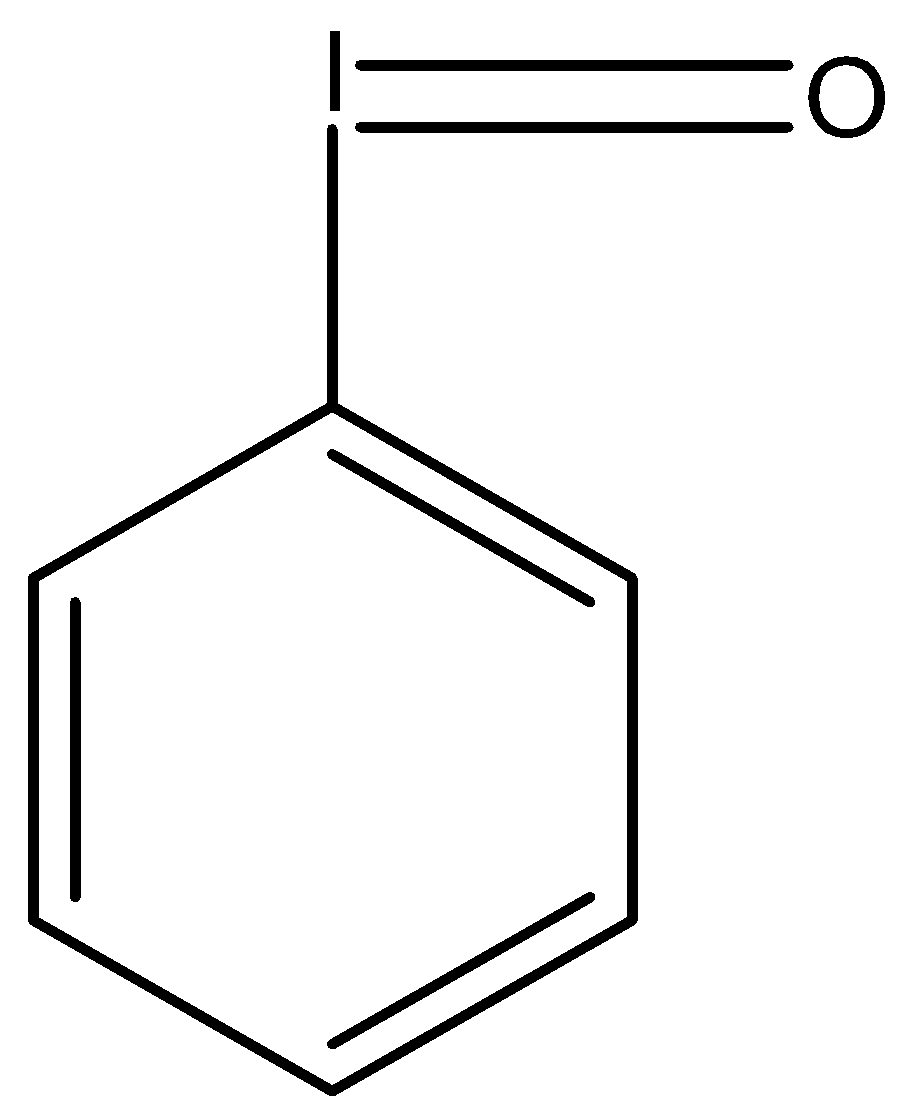
So as we see that the iodine atom is attached to the carbon atom in the benzene ring with a single bond and it is also attached with the oxygen atom with the double bond, so the hybridization will be sp2
Therefore, the correct answer is an option (b)- sp2
Note: Some other atoms like transition metals have higher hybridization like sp3d, sp3d2, etc. this is due to vacant d-orbital in transition metal. This is due to the fact that they can make coordination compounds.
