Question
Question: The hybridization of carbon atoms in C-C single bond of \(CH\equiv C-CH=C{{H}_{2}}\) is: A. \(s{{p...
The hybridization of carbon atoms in C-C single bond of CH≡C−CH=CH2 is:
A. sp3−sp3
B. sp2−sp3
C. sp−sp2
D. sp3−sp
Solution
The hybridization of the carbon atoms in organic molecules is going to depend on the number of sigma bonds and number of pi bonds attached to the carbon. The hybridization of carbon also depends on the number of hydrogens atoms attached to carbon in unsaturated compounds.
Complete step by step answer:
- In the question it was asked to find the hybridization of the carbon atoms in the C-C single bond of the given molecule.
- The given molecule is as follows.
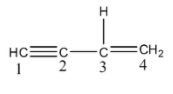
- In the given compound there is a presence of one triple bond and one double bond.
- The triple bond is present in between carbon-1 and carbon-2. The double bond is present in between carbon-3 and carbon-4. There is a single bond between carbon-2 and carbon-3.
- There is only one single bond C-C present in the given compound and it is in between carbon-2 and carbon-3.
- We can see how many sigma bonds and number of pi bonds present in the given molecule as follows.
H−spCσ≡2πspCσ−sp2CHσ=πsp2CH2
- From the above structure of the compound we can see clearly that the hybridization of carbon atoms in C-C single bond is sp−sp2 .
- So, the correct option is C.
Note: The hybridization of carbon-2 is sp because it is attached to one triple bond and the hybridization of Carbon-3 is sp2 because it is attached to one double bond.
Carbon-2 does not contain any hydrogen but carbon-3 contains one hydrogen atom with it.
