Question
Question: The hybridization and shape of \( Xe{F_2} \) is: (A) \( s{p^3} \) , bent (B) \( ds{p^2} \) , lin...
The hybridization and shape of XeF2 is:
(A) sp3 , bent
(B) dsp2 , linear
(C) sp3d2 , linear
(D) sp3d , linear
Solution
We know that in chemistry hybridization or orbital hybridization is defined as the concept of mixing or merging atomic orbitals to form a new hybrid orbital which is also known as the hybridized orbital. With the help of hybridization, we can also predict the shape or the geometry of the molecule.
Complete step by step answer:
Now we know about the basic concept of hybridization in which two atomic orbitals are combined to form a hybrid orbital. So, now we will study the hybridization of the molecule XeF2 . The hybridization of a molecule and the shape of a molecule depends on the central atom of the molecule. So, the central atom in the molecule XeF2 is xenon Xe . Now we will write the electronic configuration of Xenon which is Xe→[Kr]4d105s25p6 . The electronic configuration of the central atom helps us to find the number of valence electrons. So here according to the electronic configuration of Xenon, we can observe that it has a stable noble gas electronic configuration. So, the number of valence electrons in Xenon is 8 . Now we will study the chemical structure XeF2 .
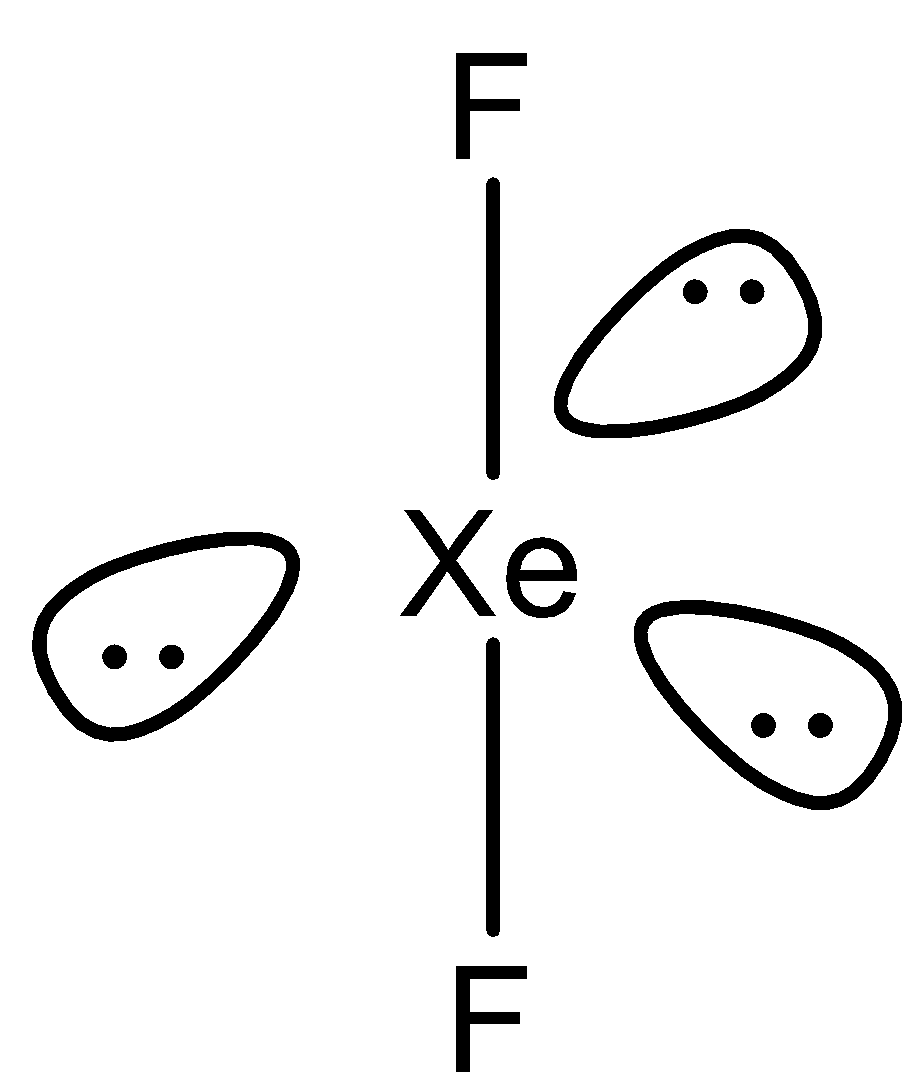
From the above structure we can observe that out of the 8 electrons, 2 electrons are bonded with fluorine atoms and the rest six electrons form three electron pairs. Now to predict the hybridization we count the sum of a total number of bonded atoms and the lone pairs over the central atom. So here, the sum is 5 . So, the hybridization XeF2 will be sp3d which means 1s , 3p and 2d orbitals are used. The shape XeF2 is linear.
Therefore, the correct option is (D).
Note:
The shape of the molecule XeF2 can be easily explained with the help of its structure. The shape is such that the lone pairs around the central atom acquire the equatorial position and the bond angle is 1800 . Therefore, the shape is linear.
