Question
Question: The hybridization and geometry of \(BrF3\) molecules are A. \(s{{p}^{3}}d\) and T shaped. B. \(s...
The hybridization and geometry of BrF3 molecules are
A. sp3d and T shaped.
B. sp2d2 and tetragonal
C. sp3dand bent
D. None of the above
Solution
Many theories have been formulated to know the nature of bonds in compounds like Valence bond theory, Molecular orbital theory, Crystal field theory, Valence shell electron pair repulsion theory.
The hybridization and geometry of BrF3 molecule is determined by VSEPR theory;i.e; valence shell electron pair repulsion theory.
Complete step by step solution:
-VSEPR theory states that the electron pairs repel each other, whether they are in the form of lone pair or bond pair. This is done in order to minimise the repulsion, thus increasing the stability of the compound.
-The knowledge of bond pair and lone pair of electrons gives us the idea of the geometry of the molecule. The geometry is found by counting the number of electron pairs and not the lone pairs.
-According to the electron pairs, the geometry can be decided as
| Number of electron pairs | Geometry of molecule |
|---|---|
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | Octahedral |
-Molecular geometry, on the other hand, also depends on the number of the lone pairs. When there are no lone pairs, the denotation is simply AXn . When lone pairs are added, the denotation becomes AXnEm
where n=no. of bond pairs
m=no. of lone pairs
-The lone pairs are placed apart from each other while the bond pairs are placed apart from each other to ensure maximum stability. So the lone pairs are always placed on the equatorial plane first.
-BrF3 molecule contains 3 bonded electron pairs as one bromine atom is bonded with 3 fluorine atoms.
-If we observe the Bromine atom, it has a total 7 electrons in its outermost shell, three of which are bonded with flourine atoms. 4 electrons remain non-bonded and thus become the 2 lone pairs of electrons. These lone pairs have to be placed on the equatorial plane only.
-F atom needs only 1 more electron to reach the configuration of Ne which it does by bonding with Br. So, it completes its octet.
So now, Br has 3 bonded electron pairs and 2 lone pairs of electrons.
-The two lone pairs occupy the equatorial plane and 1 bond pair also gets that position as there can be 3 locations on the equatorial plane and 2 on the axial plane. The remaining 2 bond pairs are located on the axial plane. Thus the shape becomes T-shaped.
-Since there are total 5 electron pairs, the hybridization is sp3d
The structure is shown as:
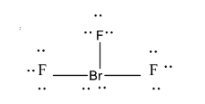
**Therefore the correct option is A.
Note: Remember that there are 3 Br-F bonds which are covalent in nature as it involves sharing of electrons. Lone pairs of electrons are 2. Also, Br is the central atom and uses d orbital for the hybridization and this is the reason why the hybridization is sp3d and not dsp3 .
