Question
Question: The hybridization and geometry of \[Br{F_3}\] molecules are: A. \[s{p^3}d\] and T- shaped B. \...
The hybridization and geometry of BrF3 molecules are:
A. sp3d and T- shaped
B. sp2d2 and tetragonal
C. sp3d and bent
D. None of these
Solution
To find out the hybridization of any molecule, one needs to find out the steric number of the central atom using its formula. The steric number is the number of sigma bonds on the central atom plus the number of lone pairs on an atom.
Complete step by step answer:
The steric number equals to 21 (number of valence electrons + number of singly bonded species with the atom whose hybridization to be determined - cationic charge on molecule + anionic charge on the molecule).
In BrF3 , the number of valence electrons of the bromine atom is 7 as its electronic configuration is [Ar]3d104s24p5 . To the central atom bromine, three fluoride atoms are singly bonded by sigma bonds. So, the number of singly bonded species with the bromine atom whose hybridization to be determined is 3. The molecule has no charge.
Putting all these values in the formula of the steric number, we get Steric number is,
21(7+3−0+0)
=21×10
=5
For molecules having steric number 5, have the hybridization of sp3d . It can either be an octahedral geometry or trigonal bipyramidal geometry. Since the molecule BrF3 has 3 sigma bonds and two lone pairs, its geometry is T shaped. The structure is shown below,
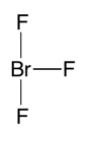
So, the correct option is A.
Note: To get the structure of any compound, one must know the number of outermost valence electrons in all the atoms and their bonding. Correct hybridization can be predicted by VSEPR theory using the number of sigma bonds and lone pairs in the compound.
