Question
Question: The hybridisation of nitrogen in pyrrole is: A.\(s{{p}^{3}}\) B.\(s{{p}^{2}}\) C.\(sp\) D. c...
The hybridisation of nitrogen in pyrrole is:
A.sp3
B.sp2
C.sp
D. cannot be predicted
Solution
The nitrogen in pyrrole contains a lone pair of electrons which is in conjugation with the double bond of the ring, which makes the compound aromatic and similar hybridisation as the carbon atom in the benzene ring.
Complete answer:
In order to understand the question, we need to learn about hybridisation:
sp Hybridisation: This is the simplest type of hybridisation involving p and s orbitals. In this hybridisation one s and one p orbitals hybridise (or intermix) to produce two equivalent hybrid orbitals, known as sp hybrid orbitals. The suitable orbitals for sp hybridisation are s and pz. if the hybrid orbitals are to lie along the z-axis. The two sp-hybrid orbitals are oriented in a straight line making an angle of 180 and therefore the molecule possesses linear geometry. Each of hybrid orbitals has 50 percent s-character and 50 percent p-character This type of hybridisation is also known as diagonal hybridisation. The two sp hybrids point in the opposite direction along the z-axis with projecting positive lobes are very small negative lobes, which provides more effective overlapping resulting in the formation of stronger bonds.
sp2Hybridisation: In sp2 hybridisation one s and two p ( and p) orbitals of one atom hybridize to give three equivalent sp2 hybrid orbitals. These three sp2hybrid orbitals are directed towards the three corners of an equilateral triangle with an angle of 1200and give a triangular geometry to the molecule.
sp3Hybridisation: In this hybridisation one s and three p-orbitals intermix to form sp3 hybrid orbitals of equivalent energy and identical shape. These four sp3hybrid orbitals are directed towards the four corners of a tetrahedron separated by an angle of 109028′. sp3 hybrid orbitals have 25 percent s-character and 75 percent p character.
Let us see the structure of pyrrole:
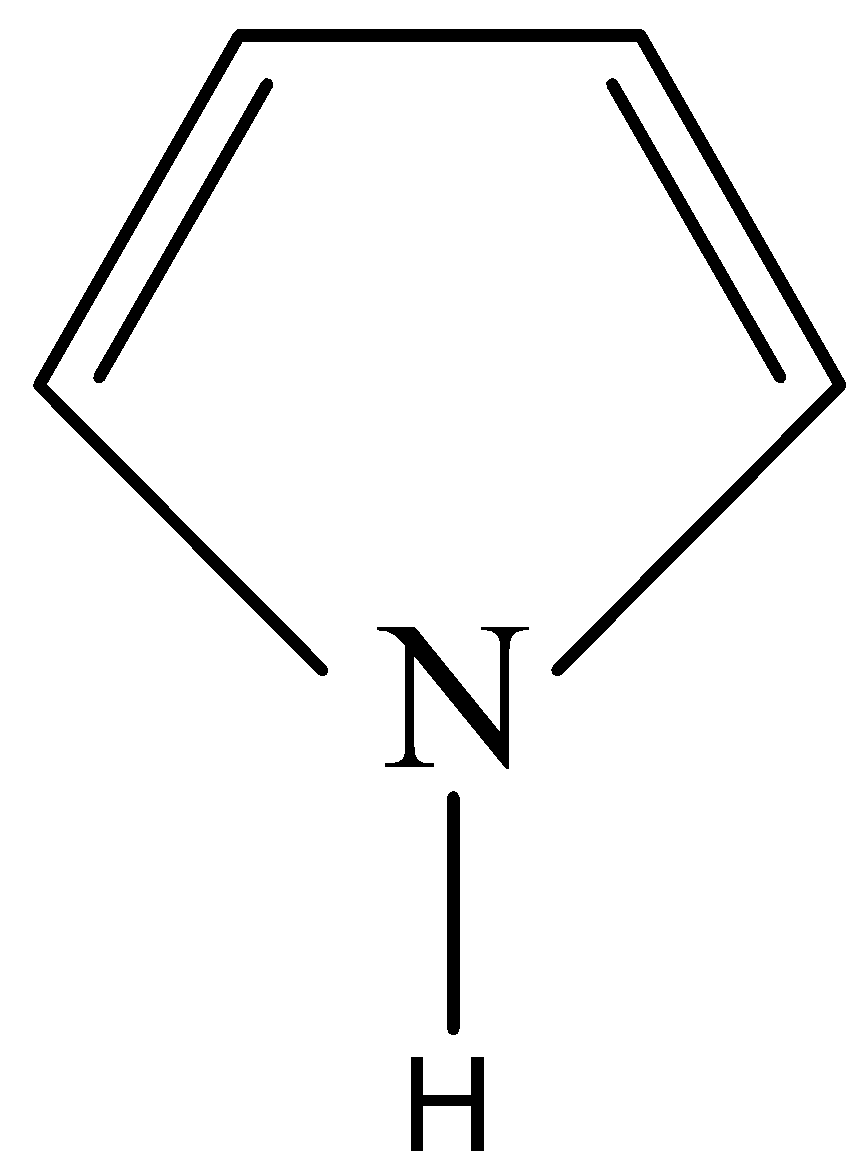
We can see that the nitrogen is sp2 hybridised.
So, we get the correct option for the question as option B.
Note:
It is to be noted that the hybridisation is not sp3, as there is a lone pair of electrons available in nitrogen which involve conjugation with the double bond.
