Question
Question: The hybridisation and geometry of \(B{{F}_{3}}\) molecule is: (A) \(s{{p}^{3}}d\) and T-shaped ...
The hybridisation and geometry of BF3 molecule is:
(A) sp3d and T-shaped
(B) sp3d2 and tetragonal
(C) sp3d and bent
(D) none of these
Solution
The Lewis dot structure is used involving the violation of octet rule. Further, to determine the shape, the orbitals in the central metal atom undergoes intermixing of its valence shell orbitals to prevent the repulsion. Thereby, predicting the shape and hybridisation of the molecule.
Complete step by step solution:
With the help of VSEPR theory, the shape/geometry of boron trifluoride can be determined. It takes into account the repulsion between the valence electron pairs in all the atoms, as they orient themselves to attain the geometry which minimises the repulsion in the resulting molecule.
- The boron atom is the central atom which is the least electronegative atom having the highest ability to share its electrons with the neighbouring atoms. Boron with atomic number = 5, has a valence shell configuration as 2s22p1, that is, three valence electrons.
-In the excited state, the one 2s electron jumps to the 2p orbital, producing three unpaired electrons. This further leads to the formation of three sp2 hybridised orbital, with equal energies and containing one electron each.
-The fluorine atom with the seven valence electrons (2s22p5). These half-filled p-orbitals of the three fluorine atoms overlap with sp2 hybridised orbitals of boron atoms, forming three coplanar B-F electron-pair bonds. Thus, having sp2hybridization.
For the Boron atom, the total number of electron pairs around it is,
=21×(no. of valence electrons + no.of atoms linked by single bonds)
=21(3+3)=3
-Then, the number of bond pairs is three which is equal to the number of atoms linked to boron atoms by single bonds.
-It has zero lone pairs as the electron pair is equal to the number of shared pairs.
Thus, we get the VSEP number equal to 3. Then, the possible shape will be trigonal planar to minimise the repulsion.
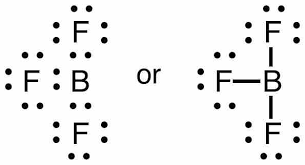
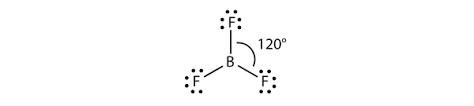
Thus, the boron fluoride molecule with sp2 hybridisation and trigonal planar geometry is an option (D)- None of these.
Note: Here, the boron atom with one empty p-orbital accepts an electron pair from any of the three fluorine atoms. Thus, forming a partial double bond and a resonance hybrid is produced. Though these forms are not readily formed.
