Question
Question: The heats of combustion of yellow phosphorus and red phosphorous are -9.19 KJ and -8.78 KJ respectiv...
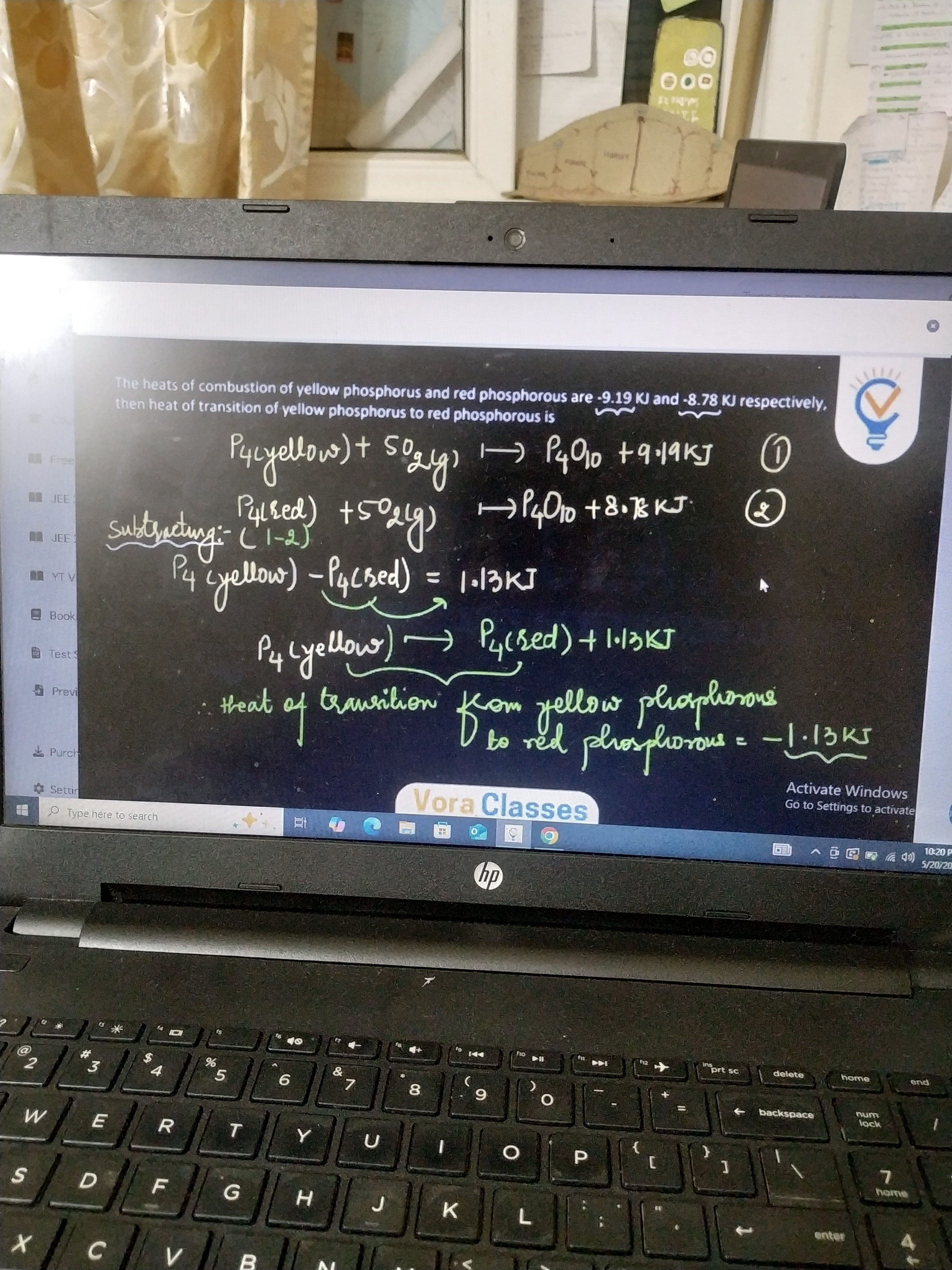
The heats of combustion of yellow phosphorus and red phosphorous are -9.19 KJ and -8.78 KJ respectively, then heat of transition of yellow phosphorus to red phosphorous is
A
- 1.13 KJ
B
- 18.69 KJ
C
- 18.69 KJ
D
- 1.13 KJ
Answer
- 1.13 KJ
Explanation
Solution
The transition reaction P4(yellow)⟶P4(red) can be obtained by subtracting the combustion reaction of red phosphorus from the combustion reaction of yellow phosphorus.
P4(yellow)+5O2⟶P4O10 (ΔH1)
P4(red)+5O2⟶P4O10 (ΔH2)
Subtracting the second from the first:
(P4(yellow)+5O2)−(P4(red)+5O2)⟶P4O10−P4O10
P4(yellow)−P4(red)⟶0
P4(yellow)⟶P4(red)
The enthalpy change for this reaction is ΔHtransition=ΔH1−ΔH2.
Using the likely intended values ΔH1=−9.91 KJ and ΔH2=−8.78 KJ:
ΔHtransition=−9.91−(−8.78)=−9.91+8.78=−1.13 KJ.
