Question
Question: The heat of hydrogenation of cyclohexene is \(28.6\;Kcal\) and that of cyclohexadiene is about twice...
The heat of hydrogenation of cyclohexene is 28.6Kcal and that of cyclohexadiene is about twice as much of cyclohexene(i.e.,55.4Kcal) . Then what would be the heat of hydrogenation of benzene, which has three double bonds?
A. Thrice that of cyclohexene (28.63Kcal)
B. The same as that of cyclohexene
C. The same as that of cyclohexadiene
D. 49.8Kcal
Solution
We are going to write down the heat of hydrogenation(△Hhyd) of each compound given along with their structure for better understanding. The compound with single double bond is cyclohexene , cyclohexadiene has two double bonds and benzene has three double bonds.
Complete step-by-step solution: In order to solve this question we are going to draw the structure of cyclohexene ,cyclohexadiene and benzene.
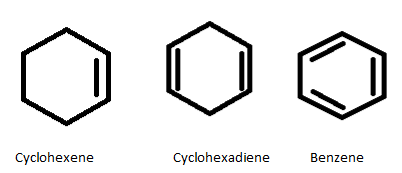
In this question we have been given the heat of hydrogenation(i.e.,55.4Kcal) of cyclohexene is 28.6Kcal and heat of hydrogenation of cyclohexadiene is twice of cyclohexene (i.e.,55.4Kcal) .
We are asked to find the heat of hydrogenation(i.e.,55.4Kcal) of benzene ,which has three double bonds.
So to proceed further, we know that,
C6H10+C6H8→C6H6
Here cyclohexene has one double bond so, counting the number of carbon and hydrogen , the molecular formula for cyclohexene is C6H10
Cyclohexadiene has two double bonds , counting the number of carbon and hydrogen , the molecular formula for cyclohexane is C6H8
Benzene has three double bonds, counting the number of carbon and hydrogen , the molecular formula for Benzene is C6H6
So adding up the amount of heat of hydrogenation we get,
28.6+55.4
Which gives =84
Which is approximately thrice of 28.6 i.e., Thrice of cyclohexene.
So among all the options, option A. is correct.
Note: Students can make mistakes in writing the correct structure of the compounds . Knowing the correct molecular formula and forming the equation of the compounds is important . Also an important concept here to keep in mind is the concept of heat of hydrogenation.
