Question
Question: The halo compound of methane used as fire extinguisher under the name pyrene is: A. \[CHC{l_3}\] ...
The halo compound of methane used as fire extinguisher under the name pyrene is:
A. CHCl3
B. CH2Cl2
C. CCl2F2
D. CCl4
Solution
In contradiction to the polycyclic aromatic hydrocarbon with the molecular formula C16H10 , the Pyrene compound in consideration in this question is halo compound of methane, which is used as a fire extinguisher.
Complete Step-by-Step Solution:
Halo compounds of methane are basically the compounds in which one or more hydrogen atoms on the carbon atom in methane get replaced by a halogen.
Pyrene is a compound which has been used for a long time as a fire extinguisher. In 1910, a certain company in Delaware had patented the use of tetrachloromethane as a fire extinguishing compound. The compound was named after the company which patented it, i.e. Pyrene Manufacturing Company of Delaware. The heat of combustion caused the liquid to vaporize and this is what extinguished the flames. When carbon tetrachloride is heated to decomposition, it will emit fumes of toxic gases like phosgene and hydrogen chloride.
The chemical formula of pyrene is CCl4 and the molecular structure of the compound can be represented as follows:
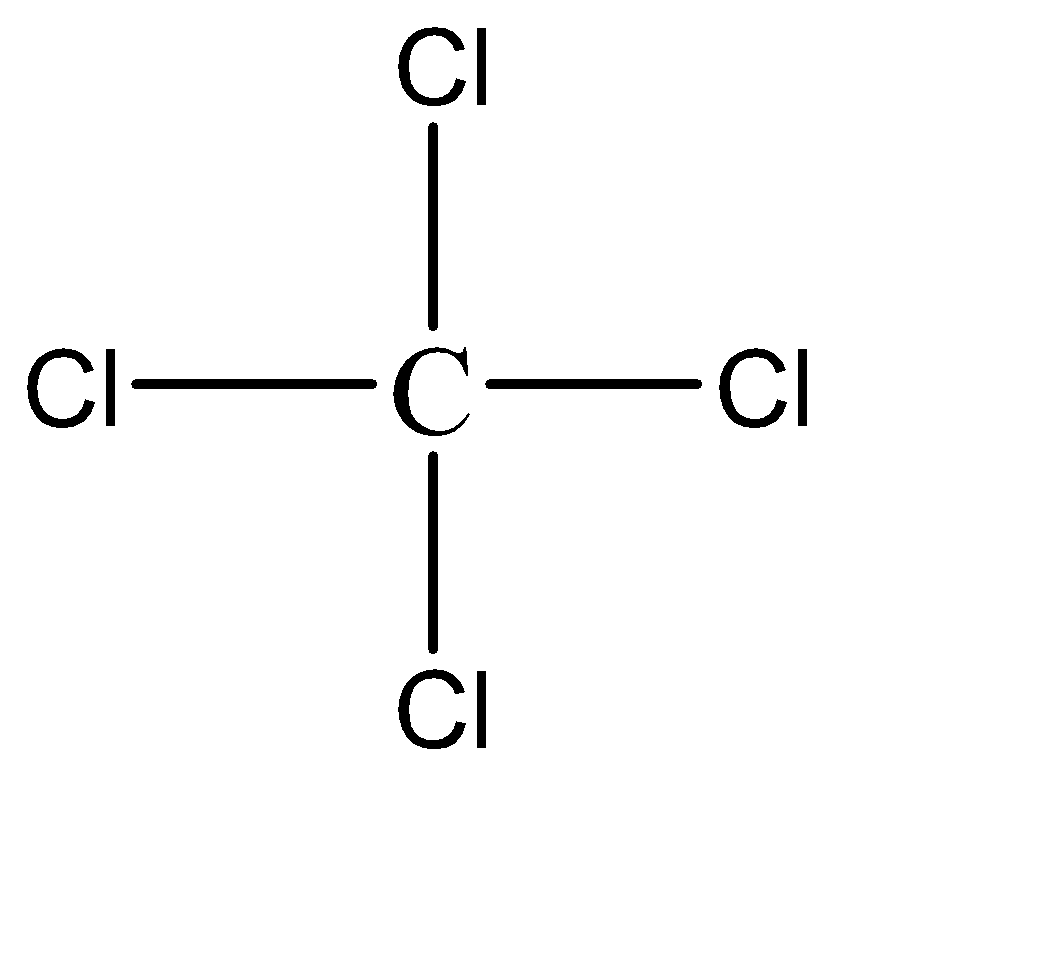
Hence, Option D is the correct option
Note: Some of the other common names used for pyrene are carbon tet (which is used in the cleaning industry), Halon (used in firefighting) and Refrigerant-10 (used in HVACR) carbon tetrachloride is used as a fire extinguisher because of two main reasons: it has no flash point and it is not flammable
