Question
Question: The \(H-C-H\) bond angle in \(C{{H}_{4}}\) is \({{109.5}^{\circ }}\), due to the presence lone pair,...
The H−C−H bond angle in CH4 is 109.5∘, due to the presence lone pair, the H−O−H angle in H2O will:
(a)- Remain same
(b)- Increases
(c)- Decreases
(d)- Becomes 180∘
Solution
If the molecule does not have any lone pair then the structure of the molecule is symmetric. Due to the symmetric structure, the bond angle remains the same. If the molecule has lone pairs, the bond pairs will experience repulsion hence the bonds will come closer.
Complete step by step answer:
The bond angle of a molecule tells the direction of the bonds.
When the molecule has a symmetric structure, i.e., they have only bond pairs. The bond angle can easily be determined. According to the hybridization of the molecule, the bond angle of the molecule can be predicted. If the molecule has sp hybridization the bond angle is 180∘, if the molecule has sp2 hybridization, the bond angle is 120∘, if the molecule has sp3 hybridization the bond angle is 109.5∘, etc.
The CH4 molecule has a symmetric structure because it has four bond pairs. The hybridization of carbon in CH4 is sp3. The bond angle between the atoms is 109.5∘. The structure of CH4 is given below:
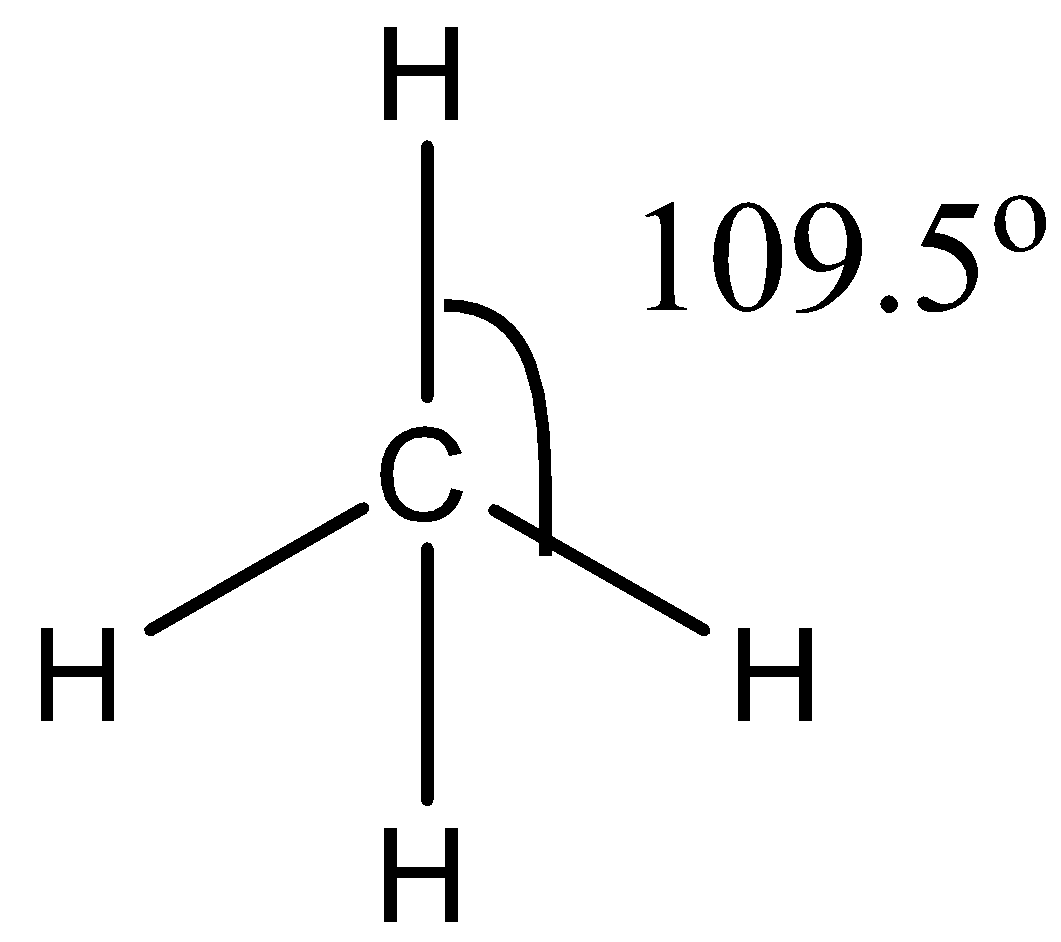
The H2O molecule has 2 bond pairs and 2 lone pairs. Due to the presence of lone pairs, the bond pairs will experience repulsion due to the bond pairs will come closer to each other. Due to this repulsion of lone pairs, the bond between bond angles becomes 104.5∘. The structure of H2O is given below:

So, the bond angle of H2O decreases.
So, the correct answer is “Option C”.
Note: Since the hybridization of carbon and oxygen in CH4 and H2O respectively same, i.e. sp3. The structure of CH4is tetrahedral. But the structure of H2O is bent or V-shaped due to the presence of 2 lone pairs.
