Question
Question: The \({H_a}\) -line of hydrogen (A) has a wavelength \(4860\,\mathop A\limits^0 \) (B) has a wav...
The Ha -line of hydrogen
(A) has a wavelength 4860A0
(B) has a wavelength 6562A0
(C) has a wavelength smaller than that of the Hβ -line
(D) is emitted in the transition from the second excited state of the first excited state.
Solution
The Balmer series includes the transition of the electrons from the higher energy levels like three or greater than three to the second energy level. If the transition takes place to the first energy level, it is included in the Lyman’s series.
Complete step by step solution:
The Hα is the visible deep red spectral line that is formed in the Balmer series when the hydrogen electron moves from the third to the second lowest energy level. It is considered as the brightest spectral line of the hydrogen that is visible. The wavelength of the Hα spectral line is 6562A0.
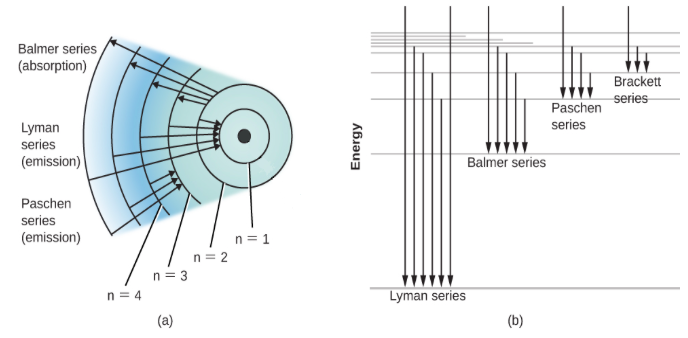
These molecules form the greater part of the nebulae and the clouds. The clouds are made up of the carbon dioxide, water molecules, Hα molecules, ammonia, formaldehyde etc. Hα helps in indicating the shape and the extent of the clouds and the other molecules help in forming the whole mass of the clouds. They have the wavelength greater than that of the Hβ line.
Thus the option (B) is correct.
Note: This Hα spectral line is more important since they are used to observe the sun’s atmosphere which includes the chromosphere and the solar prominence. By using this spectrum, the scientist and the astronomers can find the ionized hydrogen molecules present in the gas clouds.
