Question
Question: The group having triangular planar structures is: A.\({ NCl }_{ 3 },{ BCl }_{ 3 }{ ,SO }_{ 3 }\) ...
The group having triangular planar structures is:
A.NCl3,BCl3,SO3
B.CO32−,NO3−,SO3
C.BF3,NF3,CO32−
D.NH3,SO3,CO32−
Solution
Hint: In the trigonal planar arrangement, there is a central atom, bonded to three other atoms. These three other atoms are arranged like a triangle around the central atom, with a bond angle measuring 120∘C.
Complete step-by-step answer:
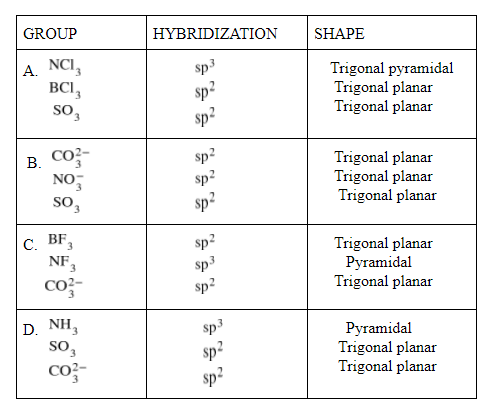
As we can see here that CO32−,NO3−,SO3
has sp2 and trigonal planar structure, so the correct option is B.
Additional Information:
-Hybridization is a process of mixing two or more atomic orbitals of an atom having comparable energy to form orbitals of equal energy known as hybrid orbitals.
-One s and three p orbitals of carbon mix/hybridize to give four sp3 hybrid orbitals. The sp3 hybrid orbitals are used by 4 hydrogen atoms for sigma bond formation.
-Trigonal planar is a subatomic geometry model with one molecule at the middle and three particles at the sides of a symmetrical triangle, called peripheral atoms, all in one plane.
- In an ideal trigonal planar species, every one of the three ligands are indistinguishable and all bond angles are 120∘C.
-When sp3 orbitals are formed, they arrange themselves with the goal that they are as far separated as could be expected under the circumstances. That is a tetrahedral arrangement, with a point of 109.5∘C.
Note: The possibility to make a mistake is that you may choose option A. But in that NCl3 is trigonal pyramidal, not trigonal planar due to the presence of lone pairs of electrons on nitrogen atoms.
