Question
Question: The ground state electronic configuration of \[{\text{CO}}\] molecule is: A.\[1{\sigma ^2}2{\sigm...
The ground state electronic configuration of CO molecule is:
A.1σ22σ21π43σ2
B.1σ22σ23σ21π22π2
C.1σ22σ21π23σ22π2
D.1σ21π42σ23σ2
Solution
For this question we must have the knowledge about making molecular orbital diagrams for hetro molecules. The electronic configuration will be written in the same way as an electron is being filled in MO diagrams from bottom to top.
Complete step-by-step answer: Molecular orbital is basically the mixing of atomic orbital. The orbits are mixed according to the principle called linear combination of atomic orbital. It states that when two atomic orbital approaches each other they interfere linearly. Every orbital has a wave function. If the two wave functions have the same phase that is plus and plus or minus and minus then they will have constructive interference. Due to this bonding molecular orbital forms which are of lower energy than the original orbital. Now if the orbital combines in an opposite phase that is plus and minus or minus and plus then they will have destructive interference. . Due to these anti-bonding molecular orbital forms which are of higher energy than the original orbital.
For mixing of atomic orbital they must have the same or comparable energy.
In MO diagram of CO , since oxygen is more electronegative so 2s orbital of oxygen has very low energy due to penetration. Hence it will directly go to non-bonding. Then first mixing will occur in 2p orbital of oxygen and 2s orbital of carbon. When two atomic orbital mixes one sigma bonding and one sigma anti-bonding orbital will form. This is an example of pre sp mixing or non ideal mixing, a sp orbital form by missing 2s orbital and 2p orbital of carbon and this orbital on interaction with one p orbital of oxygen form a non bonding. In diatomic molecules only one sigma bond can be formed which we have already formed. The two remaining p orbital will form π bonding and anti bonding molecular orbital. Hence the diagram will be like:
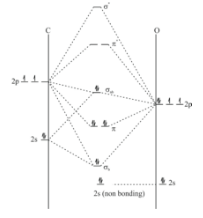
The configuration will be 1σ22σ21π43σ2
The correct option is A.
Note: The bond order of carbon monoxide is 3. When CO interacts with metal, it donates electrons to metals, but it is a pi acceptor ligand and takes electron density from metal through back bonding. Since CO has vacant anti bonding molecular orbital to accept electron and so bond order of CO reduces and become 2.5
