Question
Question: The ground state electronic configuration of \(CO\) molecule is: A: \(1{\sigma ^2}2{\sigma ^2}1{\P...
The ground state electronic configuration of CO molecule is:
A: 1σ22σ21Π43σ2
B: 1σ22σ23σ21Π22Π2
C: 1σ22σ21Π23σ22Π2
D: 1σ21Π42σ23σ2
Solution
Electronic configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Carbon monoxide is isoelectronic with nitrogen. Isoelectronic species are those species which have the same number of electrons.
Complete step by step answer:
In this question we have to find the ground state electronic configuration of CO molecule. CO Molecule is composed of two atoms of different elements. Such molecules are called heteroatomic molecules. In heteroatomic molecules intermixing of atomic orbitals only occurs when electronegativity value is similar to the two atoms. CO is composed of oxygen and carbon.
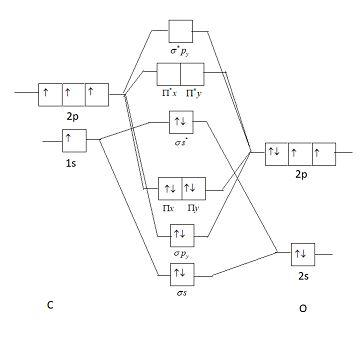
This picture shows a molecular orbital diagram of CO.
Oxygen is an electronegative element due to which it is stable and lower in energy. As energy of oxygen is lower than that of carbon, the energy difference of 2s orbital of oxygen and carbon is very high. As in heteroatomic molecules electronegativity difference should be low, electronic configuration of 2s orbital will not be considered as their mixing will be low (explained above). Electronic configuration of CO molecules is similar to nitrogen (as the number of electrons is the same in both species).
Electronic configuration of N2 is: (σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(Π2px)2(Π2py)2(σ2pz)2
Electronic configuration of CO it is 1σ22σ21Π43σ2.
Therefore the correct answer is option A.
Additional information: Bond length is approximately equal to the average distance between the nuclei of two bonded atoms. More will be the bond order more will be the pull between electrons of two atoms and less will be the bond length.
Note:
Molecules which have unpaired electrons have net magnetic moment and are called paramagnetic. A molecule which doesn’t have unpaired electrons that molecule does not have net magnetic moment and are called diamagnetic.
