Question
Question: The graph between\(\dfrac{1}{\lambda }\) and stopping potential \((V)\) of three metals having work ...
The graph betweenλ1 and stopping potential (V) of three metals having work functions ϕ1,ϕ2 and ϕ3 in an experiment of photo-electric effect is plotted as shown in the figure. Which of the following statement(s) is/are correct?[ Here λ is the wavelength of the incident ray].
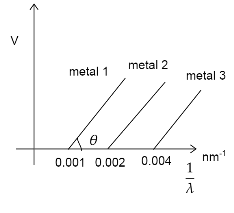
A. Ratio of work functions ϕ1:ϕ2:ϕ3=1:2:4
B. Ratio of work functions ϕ1:ϕ2:ϕ3=4:2:1
C.tanθ is directly proportional to ehc ,where h is Planck’s constant and c is the speed of light.
D. The violet colour light can eject photoelectrons from metal 2 and 3
Solution
Here we will use Einstein’s photoelectric equation. From that equation we will see how the graph between stopping potential and λ1 gives information about different parameters. From that information we will be able to answer the question. We follow these steps to answer the question.
Formula used: V0=(ehc)λ1−(ehc)λ01
Complete step by step answer:
If V0 is the stopping potential,λ is the wavelength of the incident light andλ0 is the threshold wavelength then Einstein’s photoelectric equation can be re written as
V0=(ehc)λ1−(ehc)λ01.....(1)
If we compare this equation with the equation of a straight line
y=mx+c then we can see the slope of the graph m=(ehc) . Now from the question the slope of the graph is tanθ , so tanθ=ehc .
Thus the option C is correct.
Again the equation (1) can be re written as
eV0=λhc−ϕ........(2) , where ϕ is the work function of the metal. Now when V0=0 , we can write from (2)
ϕ=λhcorϕ∝λ1
Now from the graph we have
λ11:λ21:λ31=0.001:0.002:0.004=1:2:4orϕ1:ϕ2:ϕ3=1:2:4
So the option A is correct.
Now violet has the wavelength
λv=380nmorλv1=0.0026nm−1
Thus from the graph we can see λv1>λ21 andλv1<λ31, so violet light can eject electron from metal 2 but not from metal 3 .
So option D is also incorrect.
So, the correct answer is “Option A and C”.
Note: Here we need to know about the photoelectric effect. We need to modify Einstein's photoelectric equation in different forms according to the question to be answered. An incident radiation can only eject electrons from a metal if its frequency is greater than some minimum value called the threshold frequency.
