Question
Question: The graph alongside represents a cooling curve for a substance being cooled from a higher temperatur...
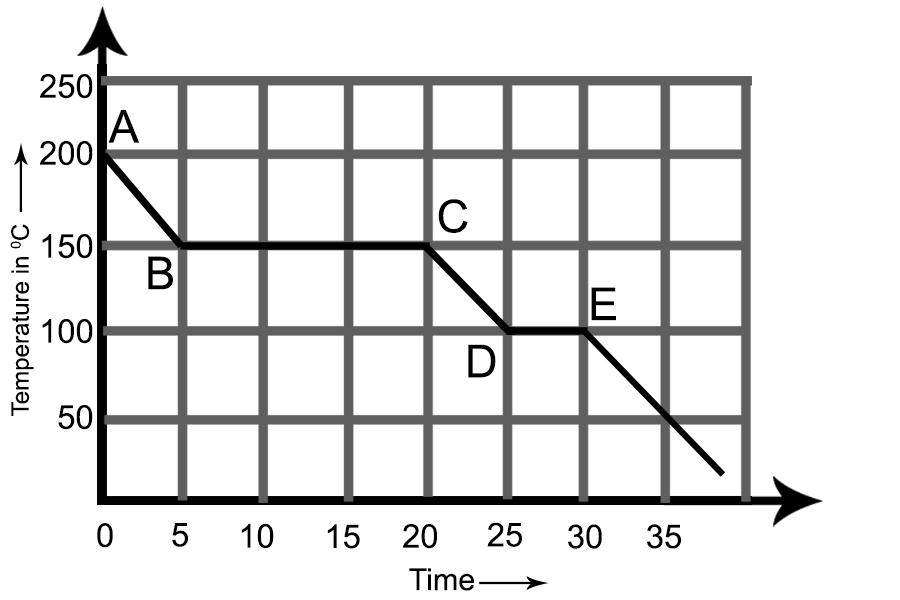
The graph alongside represents a cooling curve for a substance being cooled from a higher temperature to a lower temperature.
What is the boiling point and freezing point of the substance?
Solution
We all know that the straight horizontal lines in the cooling curve represent the release of latent heat of vaporization (BC) and latent heat of fusion (DE), respectively when a state change from gaseous to liquid and liquid to solid.
Complete step by step answer:
We have studied that the cooling curve of a substance being cooled is a temperature v/s time curve that determines the temperature change with constant cooling supplied. The straight horizontal lines are the regions when the substance's temperature remains constant despite continuous cooling provided.
The straight lines are the region where the change of state occurs. In a change of state, for example, from gaseous to liquid, the substance releases the latent heat of vaporization, which keeps the temperature of the substance being cooled at a constant value despite cooling being continuous. Similarly, during the transition from a liquid to a solid, the latent heat of fusion is released, and the temperature remains constant despite constant cooling.
By seeing at the above graph, we can show that 150 degrees are the point of boiling for this substance as it changes from the gaseous phase at 200 degrees Celsius to a vapour phase at 150 degrees Celsius. The region DE is the freezing region for this substance as this substance is changing its phase from the liquid at 100 degrees Celsius to solid.
Therefore, the boiling point and the freezing points for the substances are 150 degrees Celsius and 100 degrees Celsius.
Note: We must keep in mind that the latent heat of fusion and the latent heat of vaporization are phenomena occurring due to the change in kinetic energy of the molecules of a substance, when a substance changes state from liquid to solid, for example, the latent heat of fusion is released because some of the kinetic energy of the molecules of the liquid is lost and gets converted to heat energy and the molecules come together to form a solid structure. This is just the effect of the law of conservation of energy. Similarly, the same amount of latent heat of fusion is absorbed when a solid is heated to melt to form a liquid to facilitate the state's change, which requires the solid molecules to gain kinetic energy to spread out and the substance changes state to liquid. The temperature of the solid does not change at the melting point in a similar fashion.
