Question
Question: The given table shows the properties of four cell systems A, B, C, and D. The maximum rate of inward...
The given table shows the properties of four cell systems A, B, C, and D. The maximum rate of inward diffusion of water will be observed in which of these systems?
| System | The intracellular concentration of water | Extracellular concentration of water |
|---|---|---|
| A | 0.09M | 0.11M |
| B | 0.2M | 0.5M |
| C | 0.05M | 0.7M |
| D | 0.03M | 0.6M |
(a) System A
(b) System B
(c) System C
(d) System D
Solution
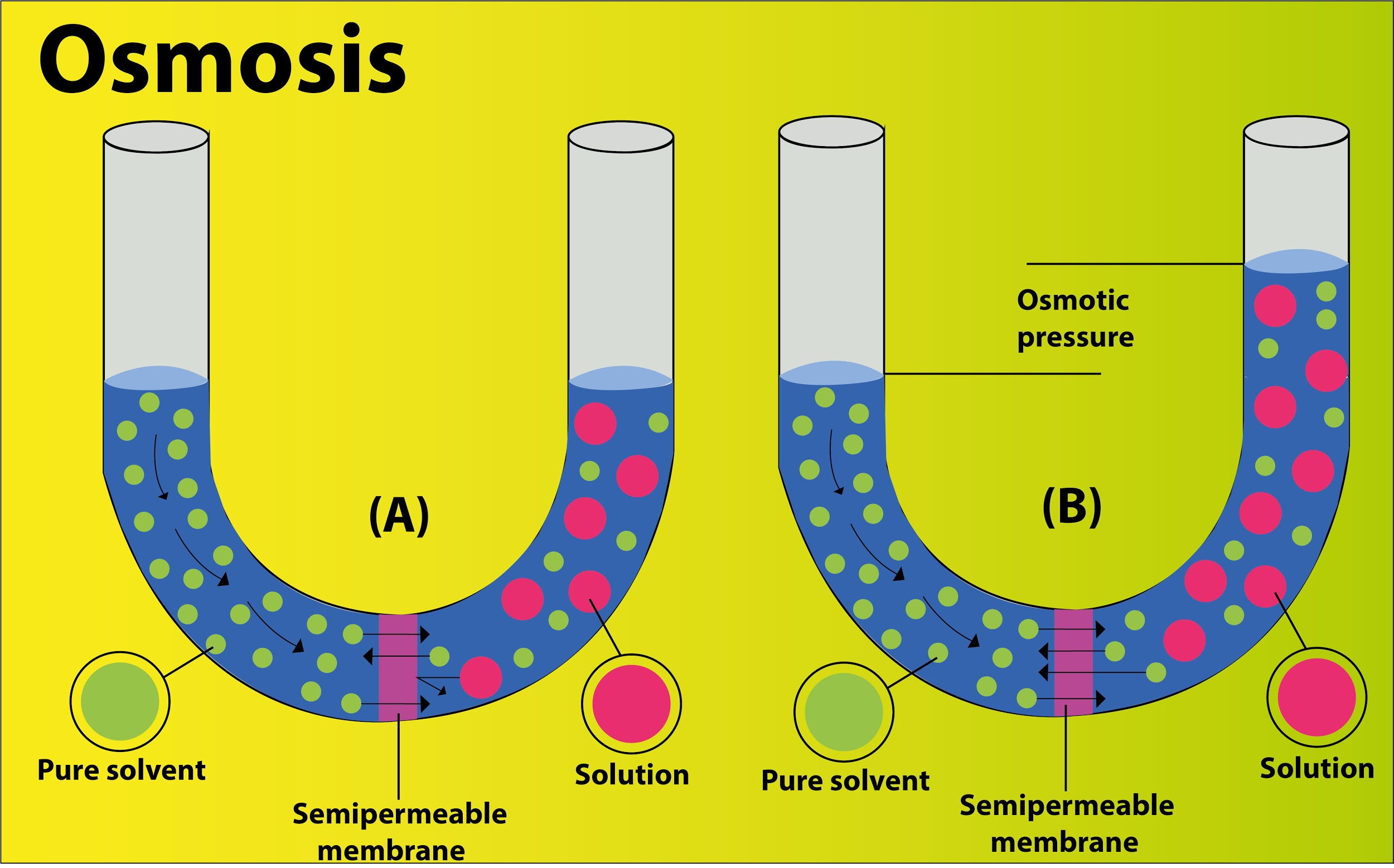
Diffusion of water across a semipermeable membrane is particularly referred to as osmosis. Movement of water molecules occurs from their higher concentration to their lower concentration until the concentration of both the solution becomes equal.
Complete step by step answer:
Osmotic pressure is the actual pressure that develops in a solution when it is separated from water by means of a semi- permeable membrane or it is the pressure needed to prevent the passage of water into the solution through the semipermeable membrane.
The more the concentration of solutes in a solution, the more pressure is required to prevent the influx of water inside and thus, the more osmotic pressure. In the given set of systems, system C has the highest difference in the concentration between the sets of solutions and thus the highest osmotic pressure due to the maximum rate of inward diffusion of water.
- Three types of solutions can be observed in the living world based on the osmotic potential- Isotonic solution, Hypotonic solution, and Hypertonic solution.
- When the concentration of the outer solution in which a cell is placed is equal to the concentration of cell sap, it is called an isotonic solution.
- When the concentration of the outer solution is higher than that of the cell sap, then such a system is referred to as a hypertonic solution.
- If the concentration of the outer solution is lower than the concentration of the cell sap, then the solution is known as a hypotonic solution.
So, the correct answer is ‘(c) System C.’
Note:
- No net movement of water across the semipermeable membrane occurs if a cell is placed in an isotonic solution and thus no change in size can be observed.
- When a cell is placed in a hypertonic solution such as salt water, the water from the cell tends to move outside. It will lead to shrinkage in cell size.
- A cell placed in a hypotonic solution will swell due to the influx of water into the cell sap.
