Question
Question: The given graphs/data 1,II,III,and IV represent general trends observed for different physisorption ...
The given graphs/data 1,II,III,and IV represent general trends observed for different physisorption and chemisorptions processes under mild conditions of temperature and pressure.Which of the following choice(s) about I,II,III and IV is(are) correct?
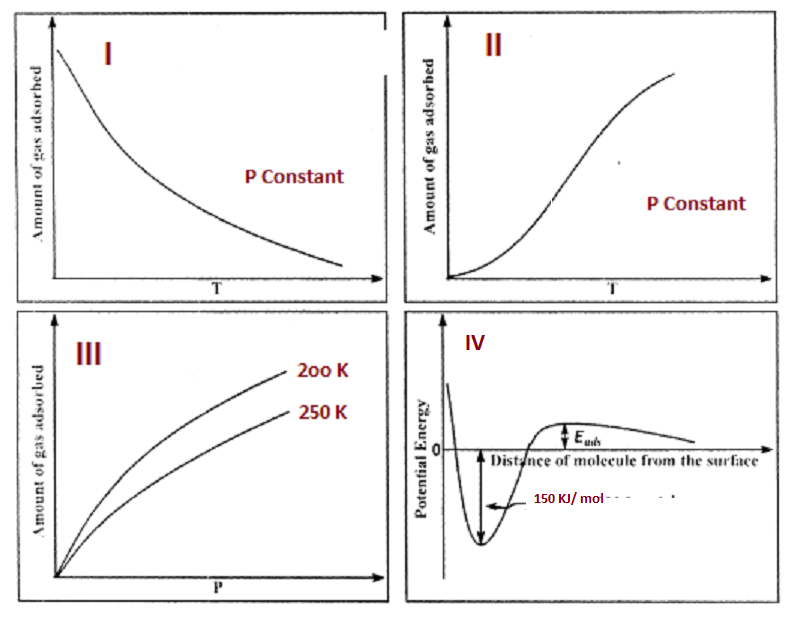
A- I is Physisorption and II is chemisorption
B- I is Physisorption and III is chemisorption
C-IV is Chemisorption and II is chemisorption
D-IV is Chemisorption and III is chemisorption
Solution
Here while solving the above graph question ,we should be fully aware of the characteristics of physisorption and chemisorption ,as there are various factors controlling it like effect of temperature, pressure, and enthalpy of adsorption.
Complete step by step solution: Let us first try to know the definition of both these process:
-Chemisorption: when the accumulation of atoms are bound to the solid surface by chemical bonds,it is known as chemical adsorption.This requires high energy and thus often referred to as activated adsorption.
-Physisorption: When the atoms are held to the surface by weak Vander waals' forces, it's known as physical adsorption.
Let's discuss few characteristics of physisorption:
- it doesn't show any specificity for a particular gas as this weak Vander waals' forces are universal.
- The enthalpy of adsorption is really low,as it is exothermic (20-40kJmol-1),due to the weak attraction between them
- Low temperature is favourable as it decreases with increase in temperature.
Now,let us know few characteristics of chemisorption:
-As it is caused by chemical bonds between the adsorbent and adsorbate,it is highly specific
-As it requires a chemical bond formation,the enthalpy is really high (80-240kJmol-1 ) and requires high activation energy sometimes.
-It favours high temperature and thus increases with increase of temperature.
-So now let us check the options:
-In the first option, it can be seen that it decreases with increase in temperature,so clearly it is physisorption.
-In the second option, the amount increases with increase in temperature,so it is chemisorption
-Now in the third option we can see that at a higher temperature,the amount is quite low,so it follows the physisorption process.
-And the fourth option clearly shows that it requires an activation energy and it has a high enthalpy of 150 kJ ,so that is favoured by chemisorption.
-So as this is a multiple correct option question:option A and C are correct.
Note: We should remember that physisorption is reversible in nature,and chemisorption is irreversible in nature as chemical bonds are involved in it.So,we should clearly try to sort out the differences between these two processes.
