Question
Question: The given conversion can be carried out using which process-  [A]i.HBr+peroxide,ii.Me3C−,iii.O3/H2O
[A]i.HBr+peroxide,ii.Me3C−,iii.O3/H2O
[B]i.HBr,ii.C2H5O−,Δ,iii.O3/H2O
[C]i.HI,ii.MeO−,Δ,iii.O3/H2O
[D]HCl+peroxide
Solution
The above conversion can be carried out by Markonikov’s addition of bromine and then Saytzeff’s elimination to remove the bromine followed by ozonolysis to cleave the bond and obtain the product. In presence of peroxide, addition is Anti-Markovnikov and in absence of peroxide, it is Markovnikov.
Complete step by step solution :
The given reaction is conversion of cyclohexanone toMeCO(CH2)4COOH.
We can carry out the following reaction in the following steps-
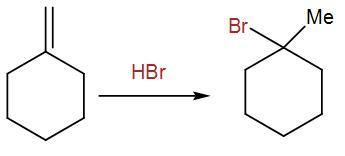
When HBr is added, it undergoes Markonikov’s addition giving us the above product.
According to the Markovnikov’s Rule, bromine will be added to the most substituted carbon, giving us the above product.
If we used HBr + peroxide, we would have got a different product as the addition would be Anti-Markovnikov’s in that case due to the peroxide effect. The bromine would be added to the least substituted carbon, hence giving us a different product.
Therefore, the first step will be the addition of bromine using hydrogen bromide in absence of peroxide.
After this, C2H5O−is added, which will give us the following product-
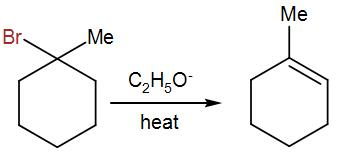
On addition ofC2H5O−, the reactant undergoes Saytzeff’s Elimination and releases bromine, leaving behind the above product.
And lastly, for the ring opening O3/H2Ois added which will break the bond at the second carbon position-
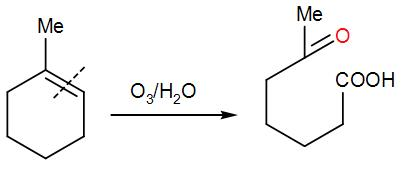
On the addition of ozone, it undergoes ozonolysis which cleaves the double bond hence resulting in ring opening and giving us the required product.
Therefore, the reagents required were i.HBr,ii.C2H5O−,Δ,iii.O3/H2O
Therefore, the correct answer is option [B]i.HBr,ii.C2H5O−,Δ,iii.O3/H2O.
Note : It is important to remember that HBr shows peroxide effect i.e.in presence of HBr and peroxide, it changes the regioselectivity and gives the opposite of the expected product, the product thus obtained is the Anti-Markovnikov product. Hydrogen chloride and hydrogen iodide do not show peroxide effect.
