Question
Question: The geometry of \(Xe{{O}_{3}}\) is ________. (A) linear (B) planar (C) pyramidal (D) T-shape...
The geometry of XeO3 is ________.
(A) linear
(B) planar
(C) pyramidal
(D) T-shaped
Solution
Start by writing electronic configuration in Xenon oxide. Calculate the number of electron pairs in the given molecule, XeO3. Find out the hybridization of XeO3 and draw the structure accordingly.
Complete step by step answer:
- Xenon belongs to the group of inert gases and it has the atomic number 54.
- Its electronic configuration is,
Xe=1s22s22p63s23p64s23d104p65s24d105p6
- Oxygen has the atomic number 8. Its electronic configuration is,
O=1s22s22p4
- For Xenon trioxide, XeO3, let’s calculate the number of electron pairs.
- Xenon has 8 valence electrons and oxygen has 6 valence electrons.
- Therefore, 8+(3×6)=226=13 electron pairs. So, XeO3 has 13 electron pairs present in it.
- Let’s draw the Lewis structure for XeO3.
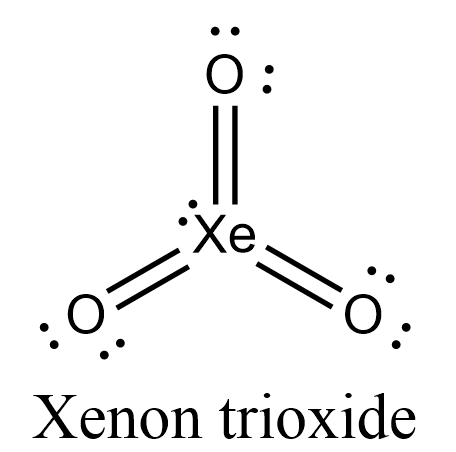
- In this structure, we can see that all 13 electron pairs are present either as bond-pairs or as lone-pairs.
- Let’s take a look at its hybridization now. Xenon has vacant 5d orbitals so, when xenon gets excited its fifth orbital configuration will become 5s25p35d3. 5s and 5p orbitals will undergo sp3 hybridization.
- Therefore, Xenon in xenon trioxide will have three sp3 hybrid orbitals, containing three unpaired electrons and one lone pair of electrons, and unhybridized 5d orbitals having three unpaired electrons.
- Oxygen will undergo sp2 hybridization and will have two sp2 hybridized orbitals having one lone pair of electrons each and one sp2 hybridized orbital containing one unpaired electron. It will have one unhybridized 2p orbital having one electron.
- The three sp3 orbitals of xenon having three unpaired electrons will axially overlap with three sp2 orbitals of three individual oxygen atoms to form three σ−bonds. The three 5d orbitals will laterally overlap with three 2p unhybridized orbitals of three oxygen atoms to form three π−bonds.
- Now, each oxygen atom has two lone pairs of electrons and a xenon atom has one lone pair of electrons. Xenon and oxygen are doubly bonded to each other.
- Due to the presence of lone pair on central atom Xenon, there will be lone pair-bond pair repulsion in the molecule. So, the trigonal planar geometry will be distorted to form pyramidal geometry.
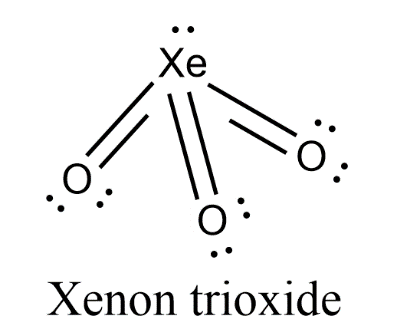
- Therefore, the geometry of XeO3 is pyramidal.
So, the correct answer is “Option C”.
Note: Remember when there is a lone pair present on the central atom, there will be lone pair- bond pair repulsion between the central atom and the other atoms linked to it. This will lead to distortion of geometry. Xenon trioxide is an unstable compound and it is a very powerful oxidizing agent.
