Question
Question: The geometry of \(\text{I}_{\text{3}}^{\text{-}}\) is: (A) Triangular (B) Linear (C) Tetrahedr...
The geometry of I3- is:
(A) Triangular
(B) Linear
(C) Tetrahedral
(D) T-shaped
Solution
The geometry of a molecule is determined according to the hybridisation of the central atom. Geometry of a molecule represents the arrangement of lone pair and bond pair surrounding the central atom.
In I3- ion, central iodine atom have sp3 hybridisation in which one s -orbital, three p-orbitals and one d- orbitals are mixed to give five new hybrid orbitals. These orbitals are equivalent in shape and energy called as sp3 hybridised orbitals. The central atom has three lone pairs and two sigma bonds.
Complete step by step answer:
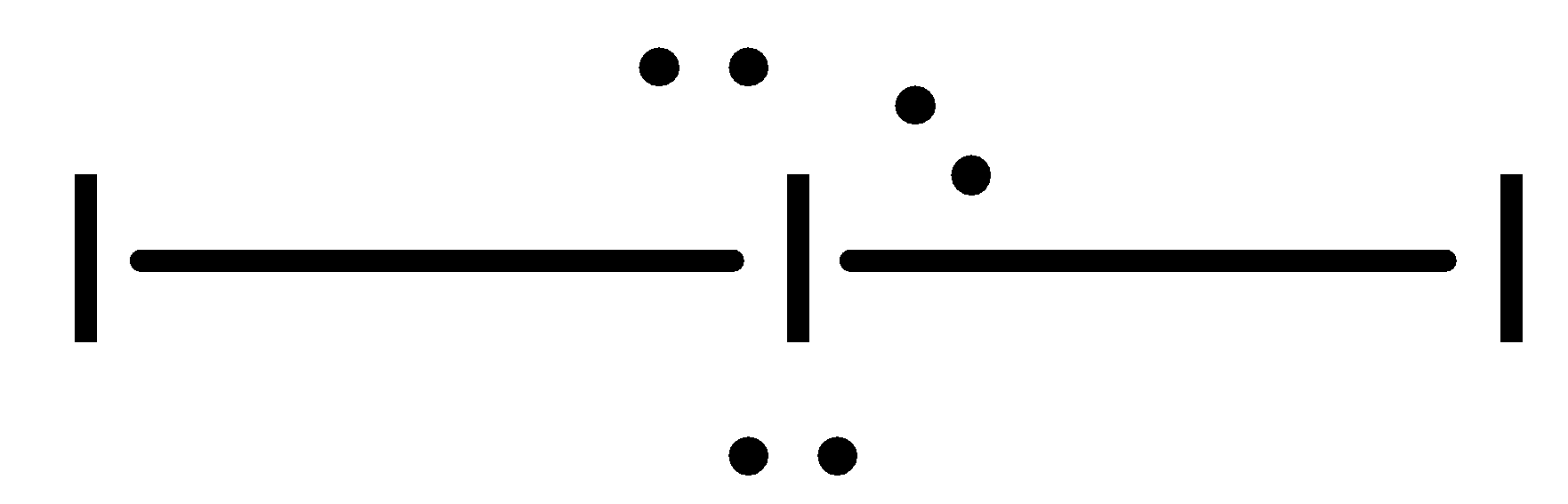
- The geometry of I3- is leaner.
- I3- Ion is made up of a I2 molecule with a I− bonded to it by mean of a coordinate bond in which I2 molecule is a lone pair acceptor or Lewis acid and I− is the lone pair donor or Lewis base.
- There are two bond pairs and three lone pairs in the outer shell of the central atom. Out of five sp3d hybrid orbitals of central atom, three are completely filled by lone pair and two are half filled which gets overlaps with two half-filled orbitals of two other iodine atoms.
- To minimise the repulsive forces the three lone pairs occupy the equatorial position. The ion is therefore linear in shape with a bond angle of exactly 180∘.
The correct answer is option “B” .
Note: If in a molecule central atom is sp3 hybridised, and have four sigma bond pair and no lone pair, the geometry of the molecule will be tetrahedral.
- If a sp3d hybridized molecule have three bond pair and two lone pair, the geometry of the compound will be ‘T’ shape.
