Question
Question: The geometry of \(Ni{{\left( CO \right)}_{4}}\) is: A. Tetrahedral B. Octahedral C. Square pla...
The geometry of Ni(CO)4 is:
A. Tetrahedral
B. Octahedral
C. Square planar
D. Pyramidal
Solution
The geometry of a complex is defined by the nature of the complex it has which certainly has an effect on the hybridization of the complex.
CO is a strong field ligand in the complex a strong field ligand induces back electron pairing from the valence shell of nickel.
Change in electronic configuration of nickel takes place when CO ligand approaches it.
Complete step by step answer:
The stable state electronic configuration of Nickel is
Argon[3d84s24p0].
The valency of Nickel is zero because in the complex, CO is a neutral ligand.
Hence, no electrons will be removed from the above stable state configuration of nickel.
CO is also a strong field ligand and when it approaches nickel, it induces back pairing of electrons from 4s to the 3d orbital emptying 4s orbital.
The new configuration of nickel becomes
argon [3d104s04p0]
And hence the 3d orbital gets filled to 10 electrons making 4s orbital and three 4porbitals ready for hybridization which decides the geometry and other important properties of the coordinate complex.
All the empty orbitals one 4s and three 4p mix and hybridize to give sp3 hybridization of the complex.
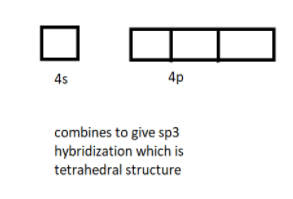
And as we know this type of hybridization corresponds to the tetrahedral structure of the complex. This is a low spin complex.
Hence the correct option for this question is A.
Note: A square planar complex is generally found to form from a strong ligand and a tetrahedral complex is generally found to be formed from weak field ligand.
The procedure used to solve the above question is done in accordance with valence bond theory.
