Question
Question: The geometry of \([Ni{{(CO)}_{4}}]\text{ and }\\!\\![\\!\\!\text{ PdC}{{\text{l}}_{4}}{{]}^{2-}}\) r...
The geometry of [Ni(CO)4] and !![!! PdCl4]2− respectively are:
a.) Both are tetrahedral
b.) Both are square planar
c.) Square planar and tetrahedral
d.) Tetrahedral and square planar
Solution
. The 3-D arrangement of atoms in a molecule is known as its molecular geometry. The molecular geometry includes the shape of the molecule as well as the bond length and bond angles of the molecule. All these factors help to determine the position of each atom in the geometry.
Complete step by step answer:
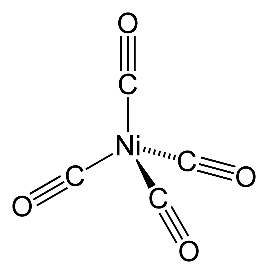
In [Ni(CO)4]
The oxidation number or Ni = 0
Electronic configuration of Ni = 1s22s22p63s23p64s23d8
And in the excited state the electronic configuration of Ni = 1s22s22p63s23p63d10
CO is a strong ligand and does the pairing which leaves an empty d orbital and in the excited state the electrons jump to the d orbitals leaving the 4s orbital empty to accommodate the electrons from CO.
Hence the hybridization is sp3 and geometry is tetrahedral
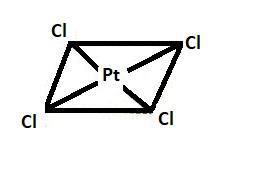
In !![!! PdCl4]2−
Electronic configuration of Pd= 1s22s22p63s23p64s23d104p65s24d8
Pd is present in +2 oxidation state here the 4d orbital of Pd gets diffused and therefore forms a strong overlap with the orbitals of the chlorine and this leads to a larger splitting in the d orbital.
Hence the geometry of !![!! PdCl4]2− is square planar.
Additional Information:
The general formula which we use to calculate the hybridization of a molecule is
Hybridization number =21[V+M−C+A]
Here V = the number of valence electrons of the central atom
C = charge on cation
A= charge on anion
M is the number of atoms linked to the central atom
So, the correct answer is “Option A”.
Note: The geometry and shape of a molecule can be the same or different as geometry of the molecule depends on the arrangement of lone pair and bond Pair while the shape of a molecule excludes the lone pair on the central atom.
