Question
Question: The geometry of a methyl carbanion is likely to be: A) Pyramidal B) Tetrahedral C) Planar D...
The geometry of a methyl carbanion is likely to be:
A) Pyramidal
B) Tetrahedral
C) Planar
D) linear
Solution
To answer this question, you should recall the formula to calculate the hybridization of a molecule. Hybridization is defined as the concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics.
Complete step by step solution:
Carbanion refers to the group of species where carbon atoms exhibit trivalence and holds a formal negative charge whose magnitude is at least -1. Carbanions usually have a bent, linear, or a trigonal pyramidal molecular geometry. An important point to remember is that all carbanions are conjugate bases of some carbon acids. In these molecules the electron density is highly concentrated at the negatively charged carbon atom. This means that this negative charge dense carbon becomes an ideal point of attack for many electrophiles and other electron-deficient species. The geometry of a methyl carbanion is likely to be pyramidal. The C atom is sp3 hybridized. There are three bond pairs and one lone pair of electrons.
This results in a tetrahedral shape or geometry and pyramidal arrangement of electrons around the central atom. Hence, the correct option is option A.
The structure of this molecule can be represented as:
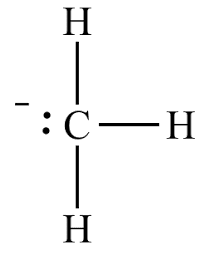
Note:
Hybridisation of a molecule can be found out using (X) using the formula: 21(V+H−C+A) where
V = Number of valence electrons in the central atom
H = Number of surrounding monovalent atoms
C = Cationic charge
A = Anionic charge. The value of X will determine the hybridisation of the molecule. If X is 2 then sp ; is 3 then sp2 ; is 4 then sp3 ; is 5 then sp3d ; is 6 then sp3d2 ; is 7 then sp3d3 hybridization.
