Question
Question: The geometrical isomerism is shown by: A. 
B. 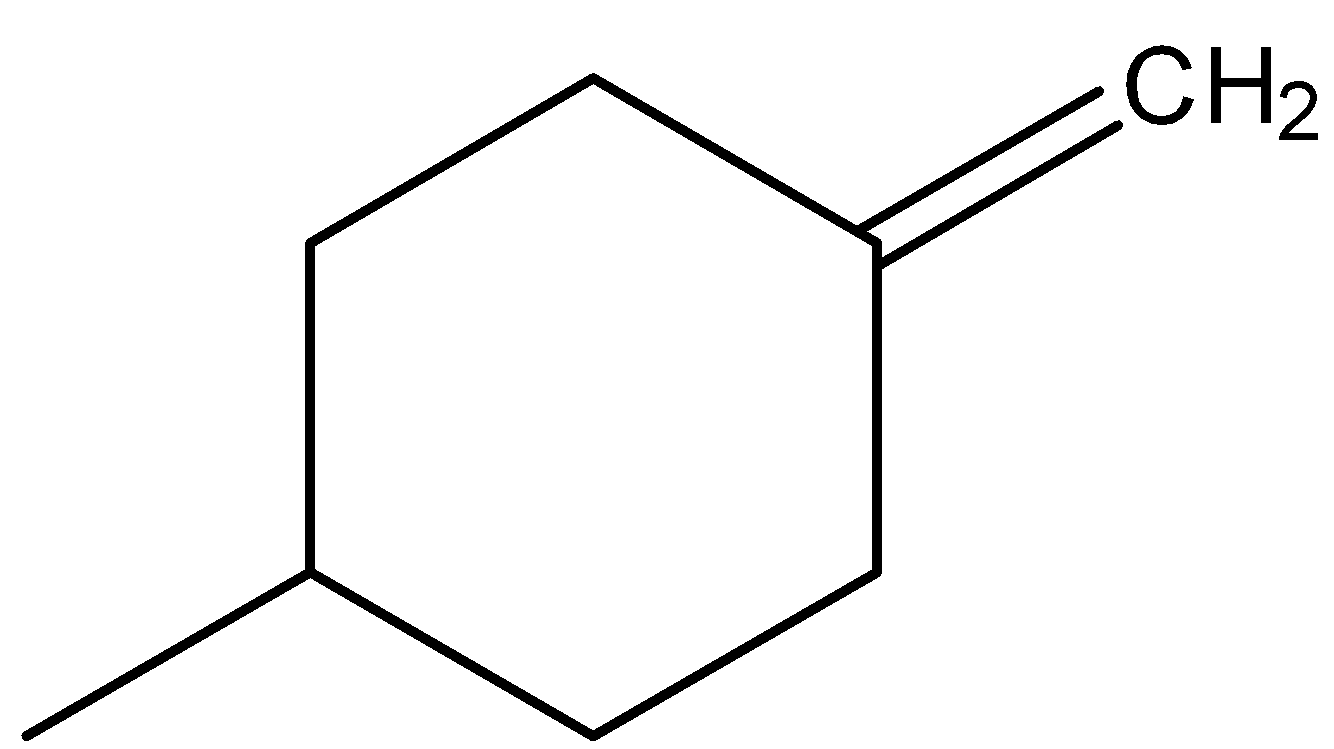
C. 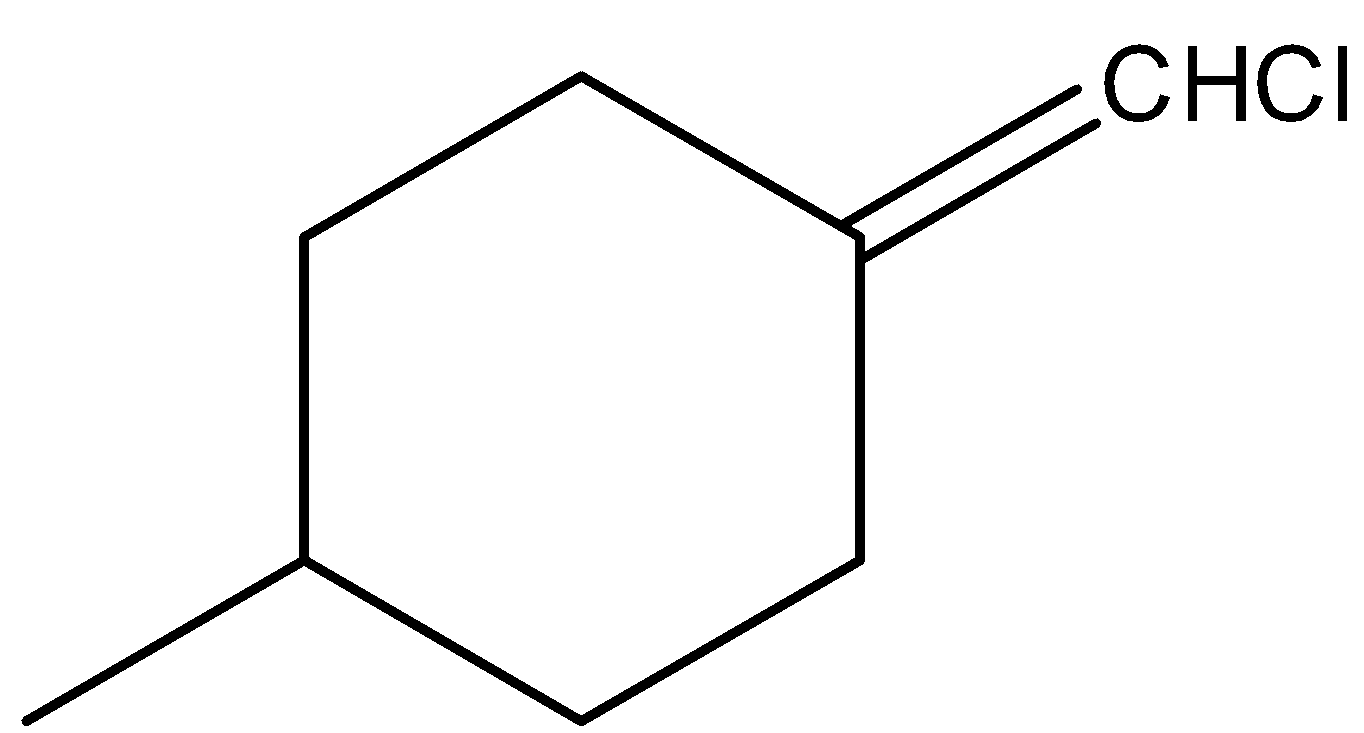
D. 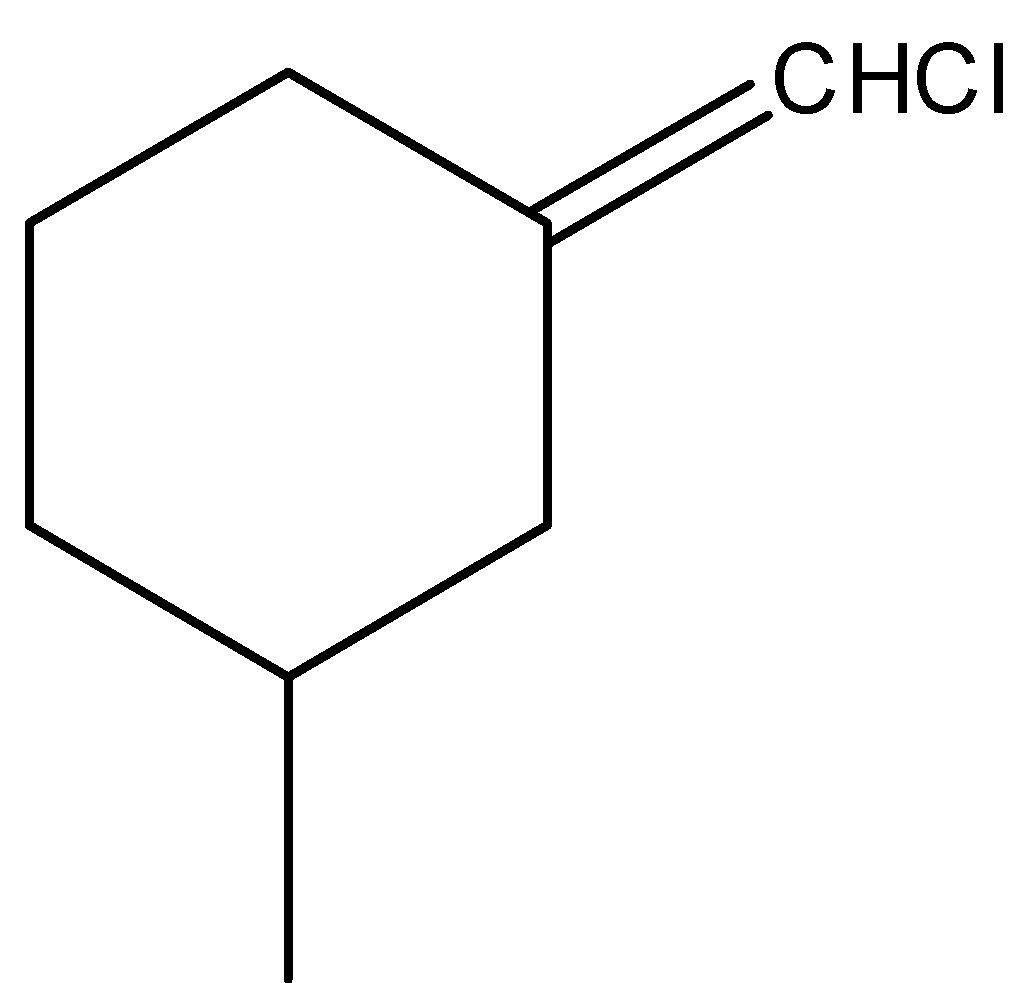
Solution
Geometrical isomerism is also called as Cis-Trans isomerism or configurational isomerism. Geometric isomers (cis and trans) have the same molecular formula but differ in the arrangement of atoms at double bonds in aliphatic or in cyclic compounds.
Complete step by step answer:
-Coming to the given options, option A,
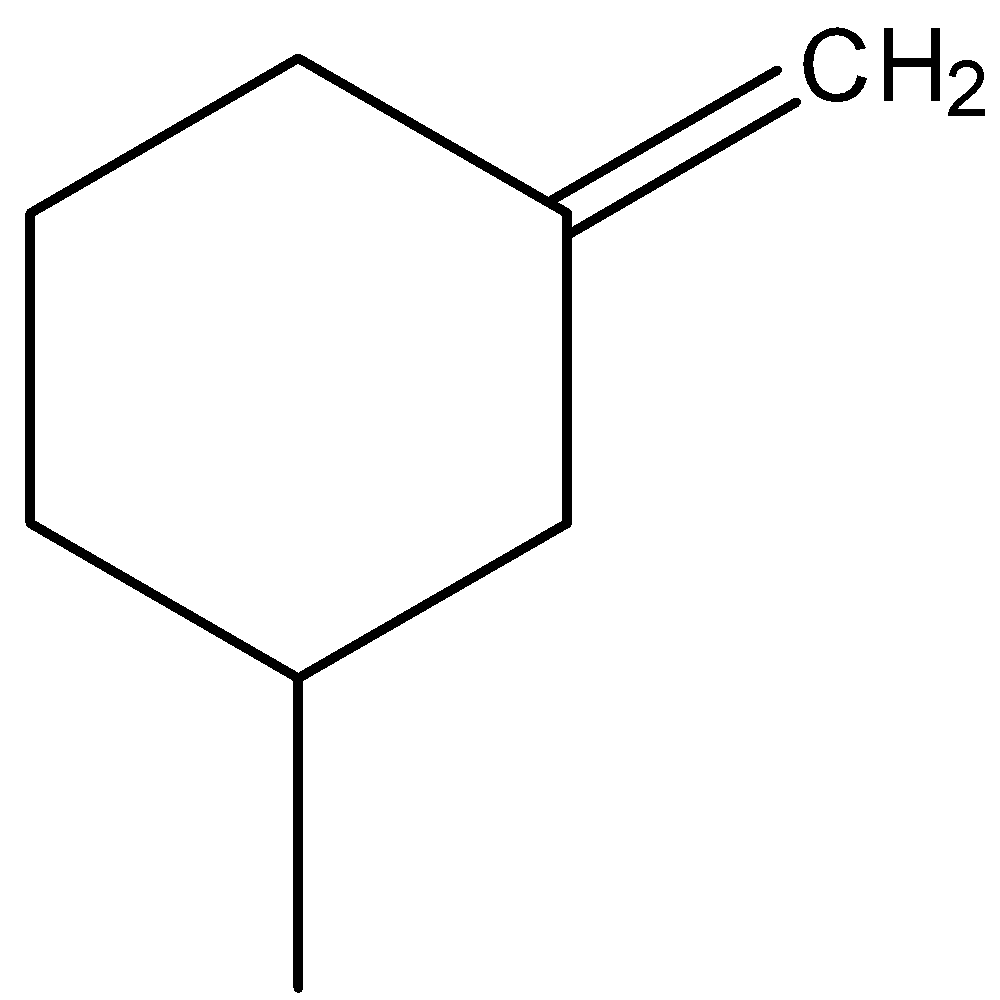
-In the above compound the atoms attached to carbon (at double bond) are the same (two hydrogens).
-So, option A won’t show any geometrical isomerism due to the absence of two different atoms at double bonded carbon.
-Coming to option B,
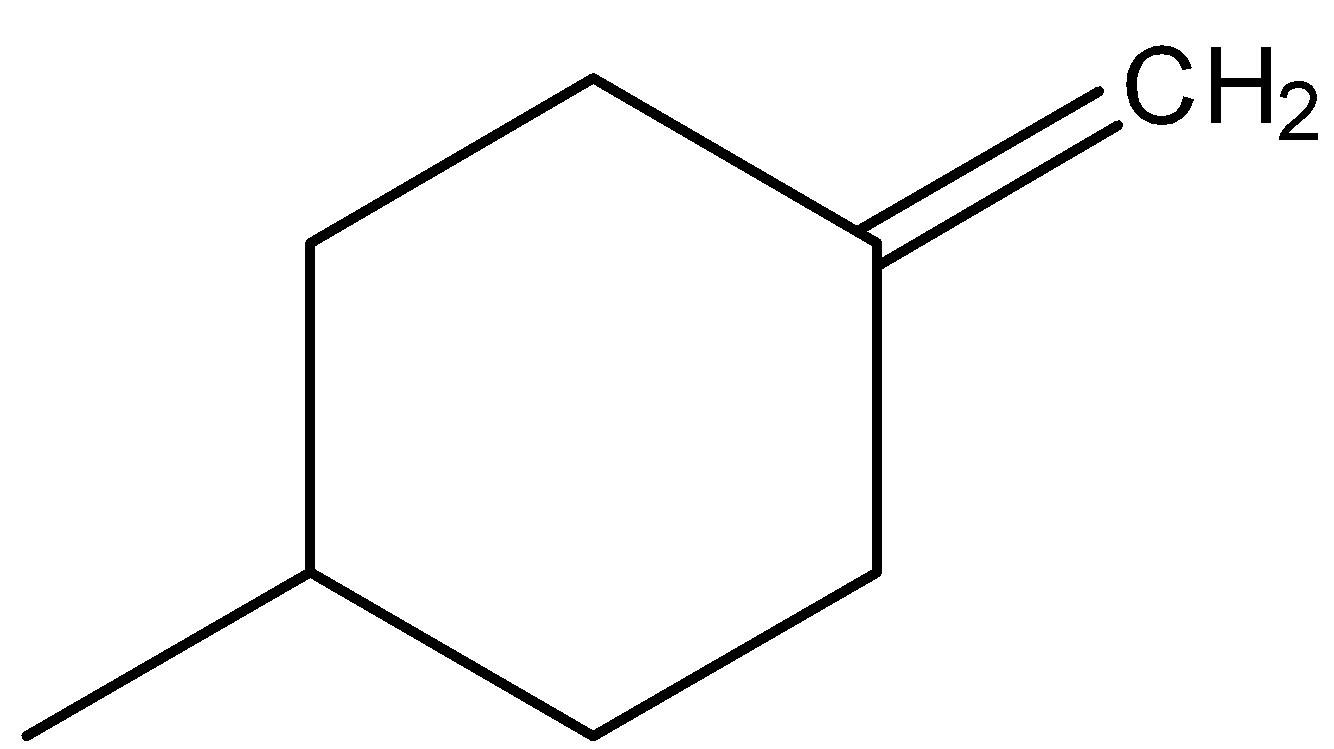
- In the above compound the atoms attached to carbon (at double bond) are the same (two hydrogens).
-So, option B won’t show any geometrical isomerism due to the absence of two different atoms at double bonded carbon.
-Coming to option C,
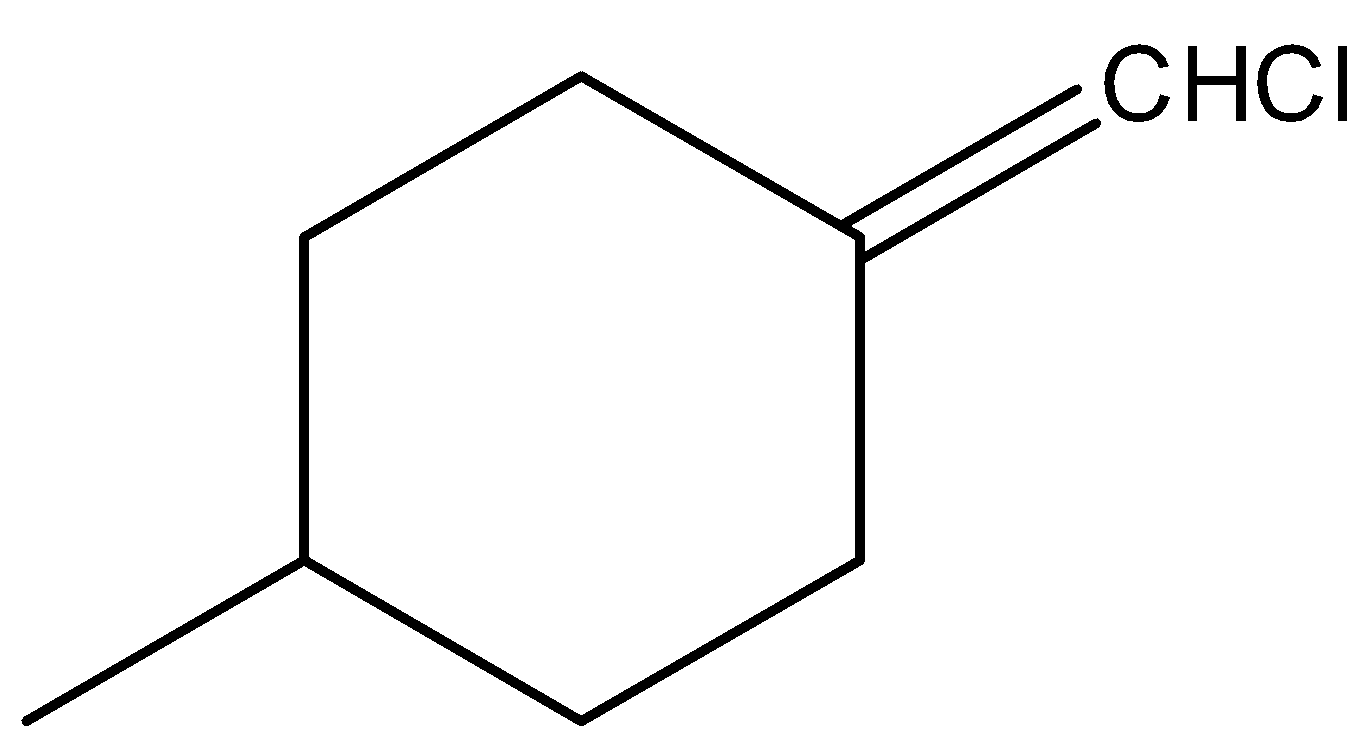
-In the above compound there are two different atoms (H and Cl) that are attached to double bonded carbon. But still the above structure does not show geometrical isomerism due to the presence of a plane of symmetry.
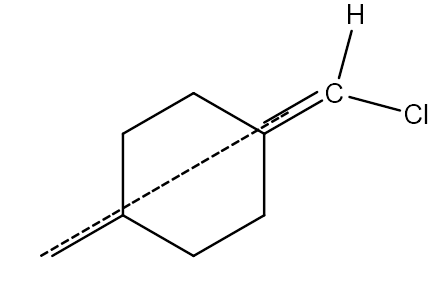
-Coming to the option D,
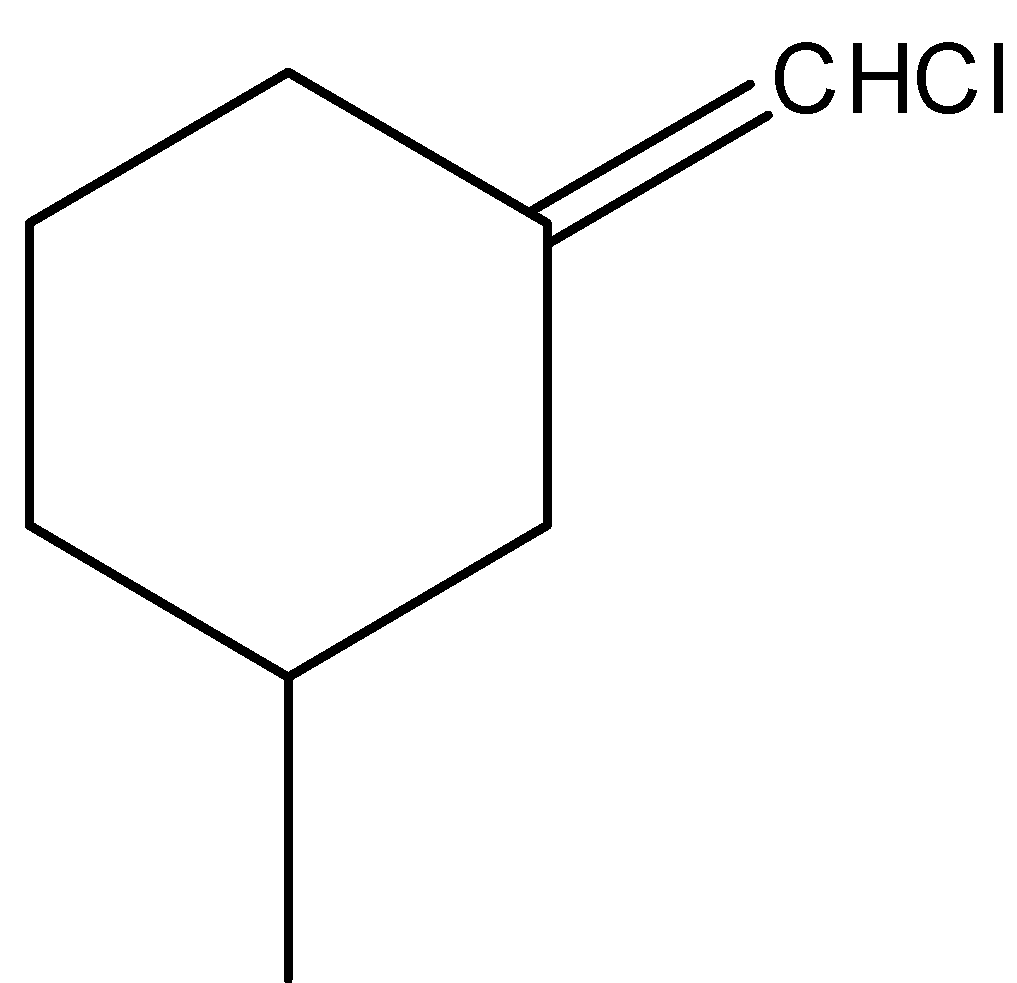
-The compound in option D shows geometrical isomerism due to the presence of double bond and different atoms attached to double bonded carbon.
-The following are the cis and trans isomers of the compound in option D.
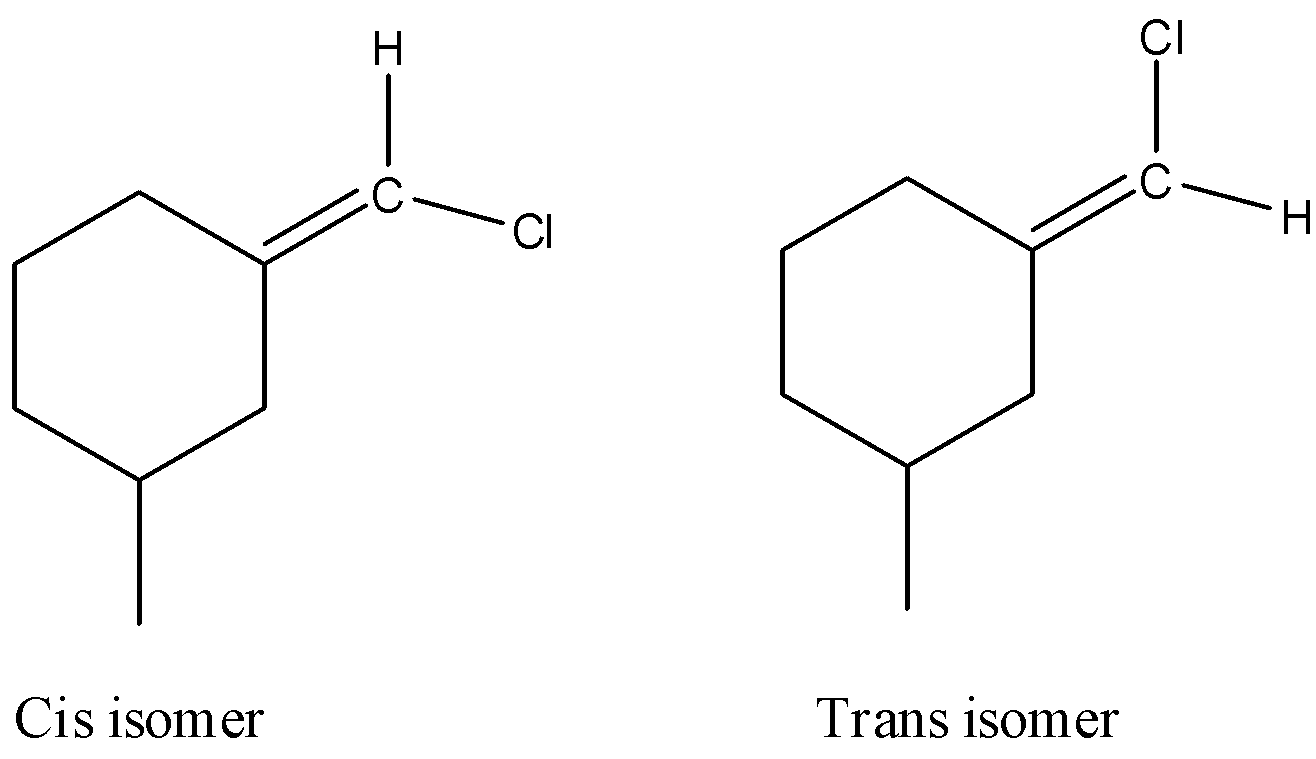
So, the correct answer is “Option D”.
Note: To exhibit geometrical isomerism the organic compounds should have a double bond (C = C) and the atoms attached to carbon atom in a compound should be different
Therefore, geometrical isomerism is going to be exhibited by the alkenes due to the presence of non-rotating double bonds.
