Question
Question: The geometrical arrangement and shape of \[\text{I}_ {3} ^ {-} \] are respectively: (A) trigonal b...
The geometrical arrangement and shape of I3− are respectively:
(A) trigonal bipyramidal geometry, linear shape
(B) hexagonal geometry, T-shape
(C) triangular planar geometry, triangular shape
(D) tetrahedral geometry, pyramidal shape
Solution
We can calculate the structure or the geometry and the shape of the I3− molecule by knowing its hybridization (it is the process of inter-mixing of the orbitals to form new orbitals of equivalent energy). The hybridization can be calculated by the formula:
H= 21 (V+M−C+A)
Here, V represents the number of electrons in the valence shell of the atom, M represents the monovalent atoms attached to that atom and C and A represents the charges of the cations and anions. Identify the structure.
Complete step by step solution:
To know the structure and shape of any molecule, we should know its hybridization. By the term hybridization, we mean the phenomenon of inter-mixing of the orbitals of slightly different energies so as to redistribute their energies and to give a new set of orbitals of equivalent energies and shape.
First, we have to find the hybridization of I3− molecule by the formula as:
H= 21 (V+M−C+A) ---------(1)
Here, V= number of the valence electrons in the atom, M= number of the monovalent atoms bonded to the central atom, C=the charge on the cation and A= the charge on the anion.
So, in case of the molecule; V in I=7
M =2
A=1
Put all these values in equation (1), we get
H= 21 (7+2+1)
= 21 (10)
=5
So, the I3−involves the sp3d hybridization i.e. it has trigonal bipyramidal geometry or the structure and it involves the mixing of the one s, three p and one d-orbitals but its shape is linear because it consists of three iodine atoms and the central iodine atom ion it consists of three lone pair of electrons and two bonds pair of electrons with the two other iodine atoms as;
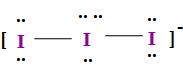
So, thus the I3− has linear shape.
Hence, the option (A) is correct.
Note: For the orbitals which undergo hybridization, they should have only small differences in their energies and only those orbitals are hybridized which are present in the valence shell of the atom.
