Question
Question: The general molecular formula of nitroalkanes is...
The general molecular formula of nitroalkanes is
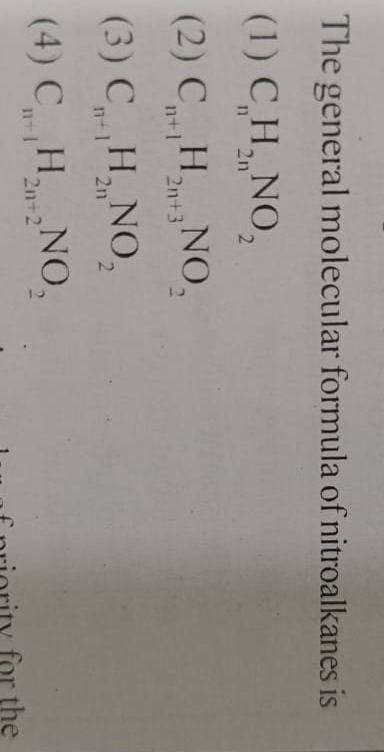
A
CnH2nNO2
B
Cn+1H2n+3NO2
C
Cn+1H2nNO2
D
Cn+1H2n+2NO2
Answer
Cn+1H2n+3NO2
Explanation
Solution
The general formula for an alkane is CxH2x+2. A nitroalkane is formed by replacing one hydrogen atom with a nitro group (-NO₂). Therefore, the general formula for a nitroalkane with 'x' carbon atoms is CxH2x+1NO2.
Option (2) is Cn+1H2n+3NO2. If we let the number of carbon atoms be x=n+1, then n=x−1. Substituting this into the hydrogen count: 2n+3=2(x−1)+3=2x−2+3=2x+1.
So, option (2) can be rewritten as CxH2x+1NO2, which is the correct general formula for nitroalkanes.
