Question
Question: The general formula \({C_n}{H_{2n}}\)is valid for alkene with ___. A. \(2\) double bond B. \(1\)...
The general formula CnH2nis valid for alkene with ___.
A. 2 double bond
B. 1 double bond
C. 3 double bond
D. None of these
Solution
We have to remember that alkenes are acyclic (branched hydrocarbons or unbranched hydrocarbons), characterized by the presence of a carbon-to-carbon double bond. The general molecular formula for alkene is CnH2n. Alkenes contain two hydrogen atoms less than the parent alkanes (saturated aliphatic hydrocarbons), so they are said to be unsaturated.
Complete step by step answer:
In the chemistry of compounds, alkenes are big different to other compounds, because of the one double bond.
The simple hydrocarbons alkanes, alkenes and alkynes should not be confused. Alkanes are the simplest organic compounds and have only single bonds and also called saturated hydrocarbons.
We have to remember that alkenes are unsaturated hydrocarbons with one carbon-carbon double bond. Alkynes are linear unsaturated acyclic hydrocarbons and contain carbon-carbon triple bonds. Alkyne is also called unsaturated hydrocarbons.
The general molecular formula for alkanes, alkenes and alkynes are CnH2n+2,CnH2n and CnH2n−2.
We have to remember that alkenes are open chain hydrocarbons and are also called olefins, some of earliest products derived from alkenes were oily (from crude oil) in appearance. So called olefins. Due to the presence of double bond alkenes are more reactive than the alkanes.
The general molecular formula CnH2nis for the homologous series of alkenes, having one carbon-to-carbon double bond (C=C).
For example, the first few alkenes are given below,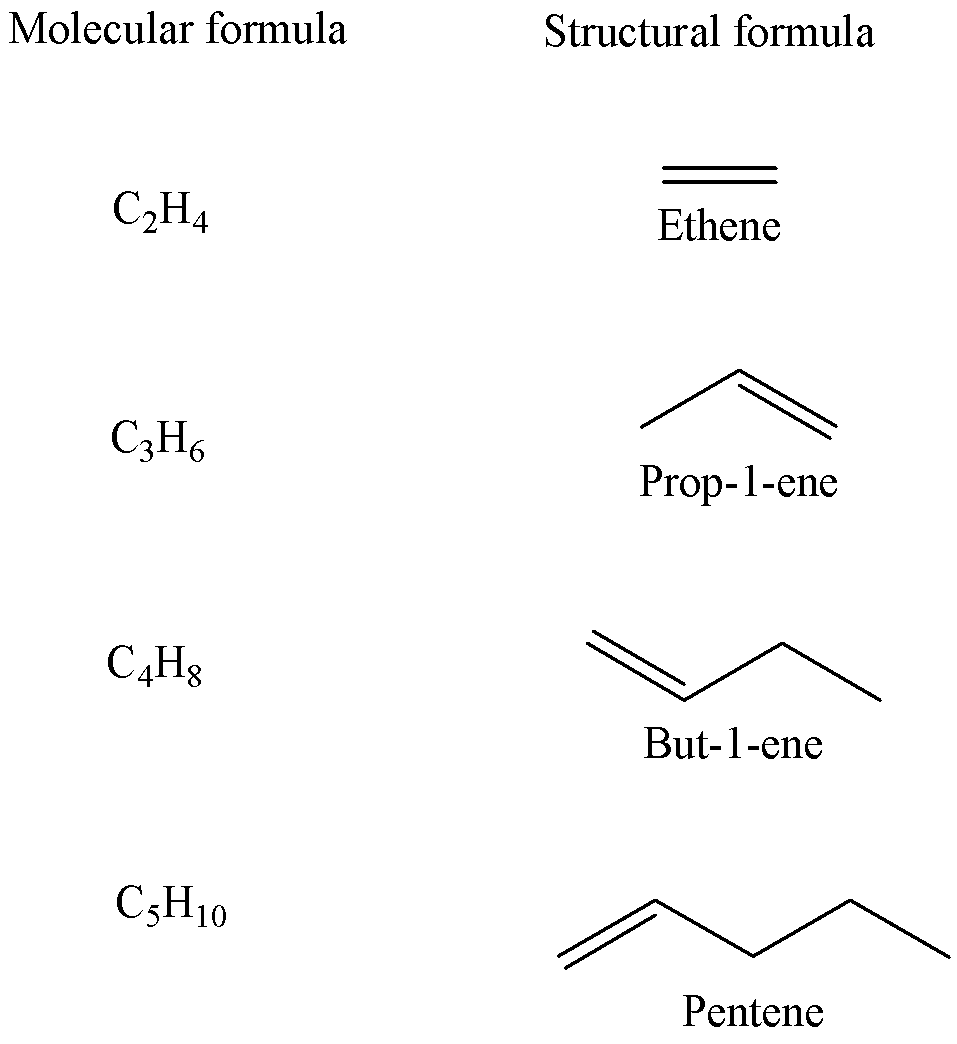
So, the correct answer is Option B.
Note: We have to remember that the alkenes have major commercial uses of production of polymers and important starting material in many consumer chemicals used in a variety of many consumer products. And also a major place in the biological world and also perfumes, flavors and fragments.
