Question
Question: The functional group in alcohol is. A. 
B. 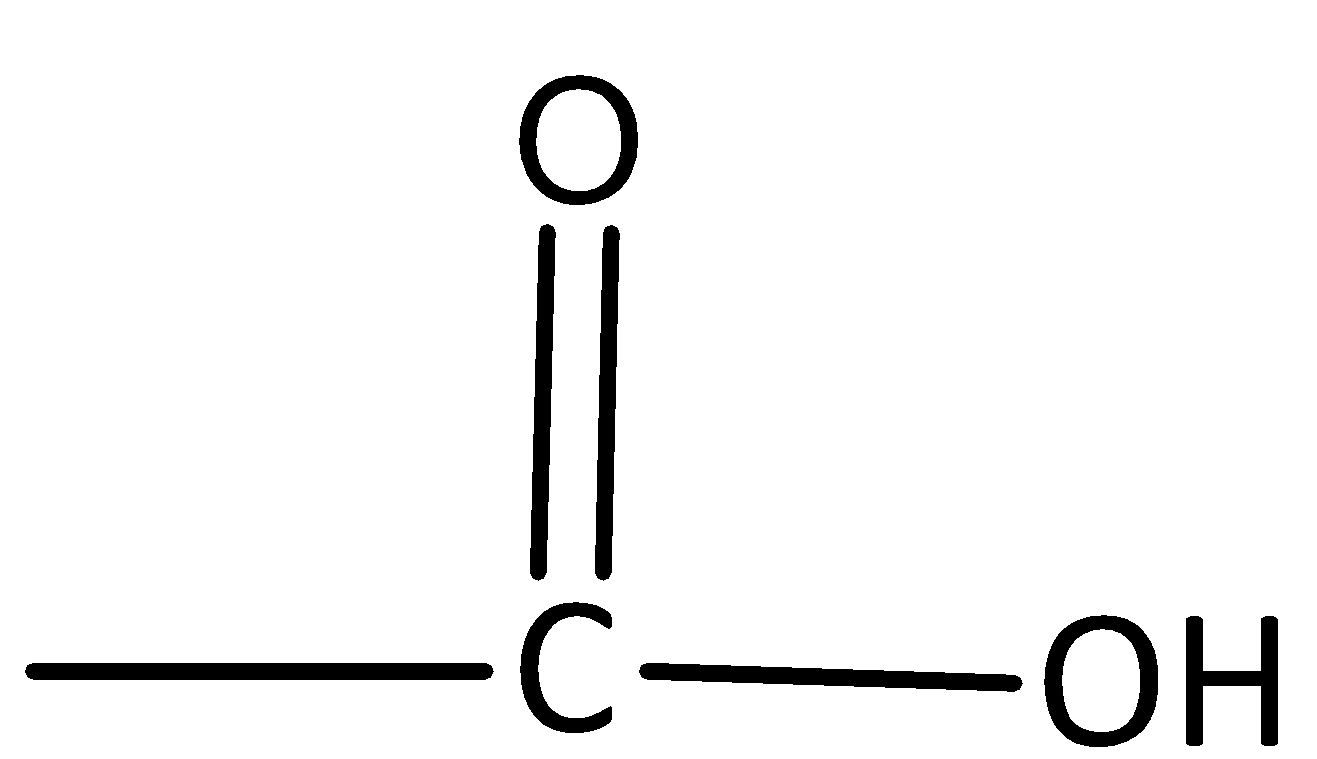
C. 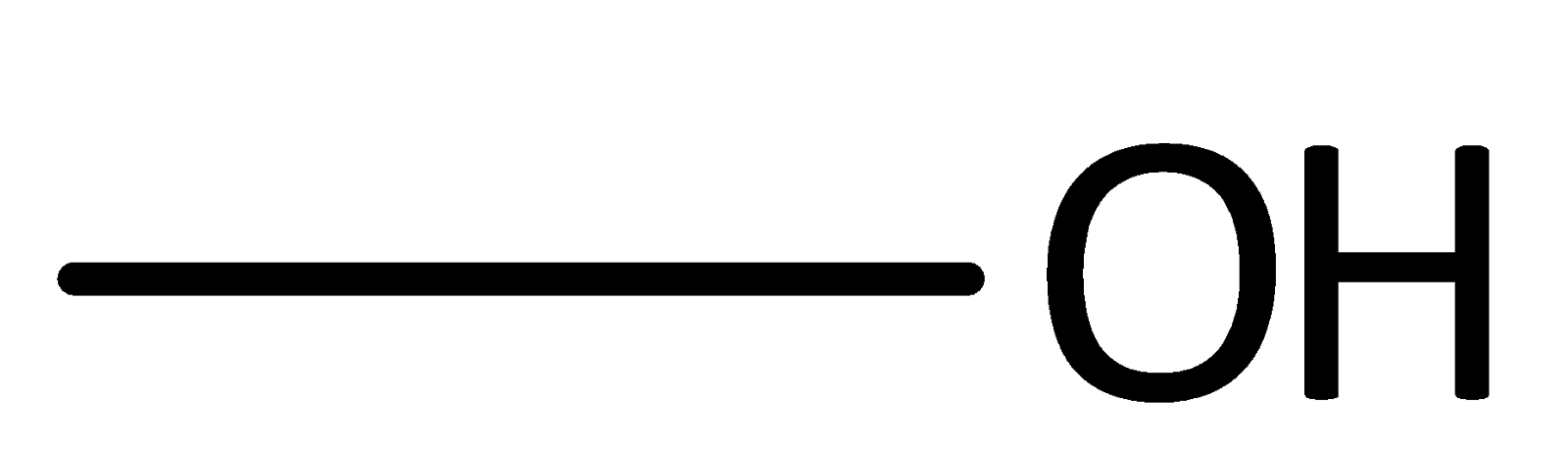
D. 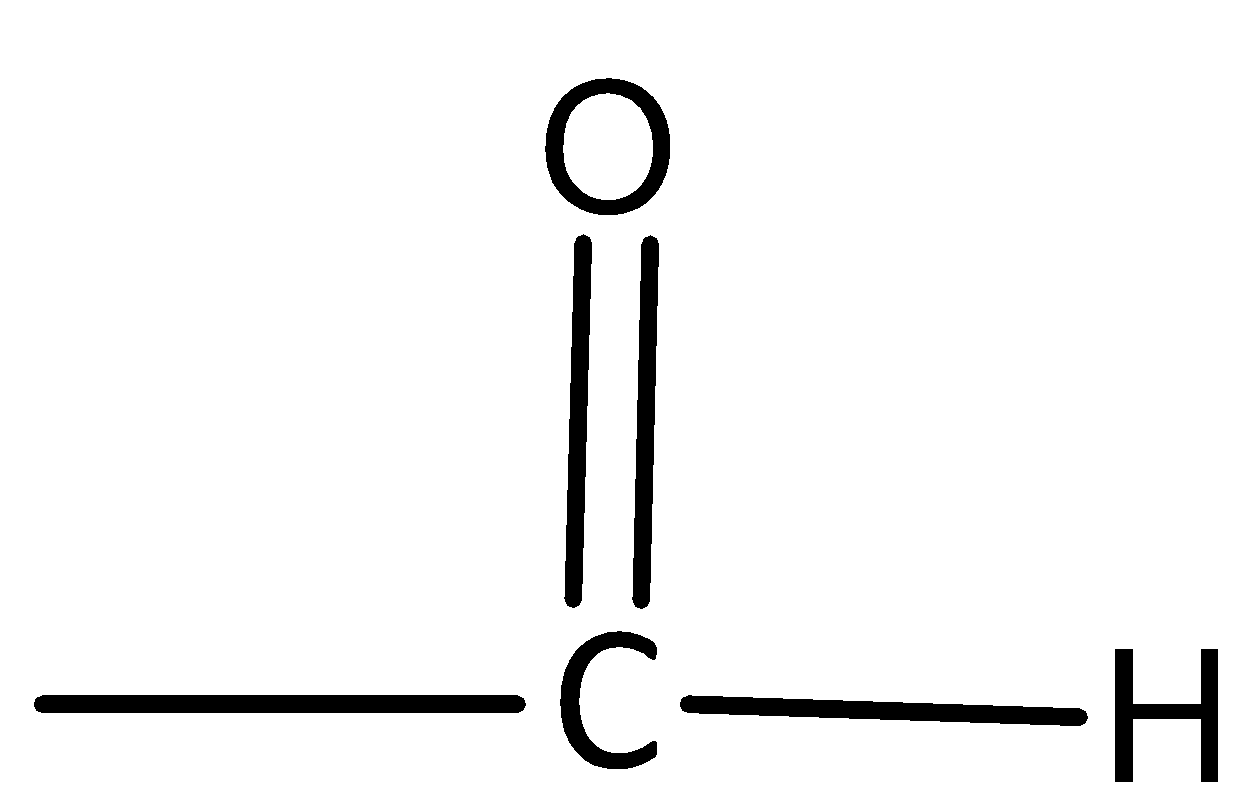
Solution
We see about the functional group.
Functional group:
We must remember that the functional group is an atom or a group of atoms that determine the characteristic property of the organic compounds despite the size of the molecule members of the same functional group undergo the identical chemical reaction.
Complete step by step answer: Let us see the functional group in detail.
A functional group may be a specific group of atoms or bonds with a compound that is liable for the characteristic chemical reactions of that compound and the molecules with the same functional group undergo similar reactions. The functional group plays a major role in the nomenclature of an organic compound.
The atoms in the functional group are linked together and to the compound through covalent bonds. An alpha carbon is the first carbon atom that attaches with the functional group and the second carbon is called beta carbon. Depending on the attached carbon functional groups are mentioned as primary, secondary, and tertiary carbon atoms.
Now, we see details about an alcohol functional group.
An organic compound that contains a hydroxyl group with the central carbon atom is called an Alcohol group.
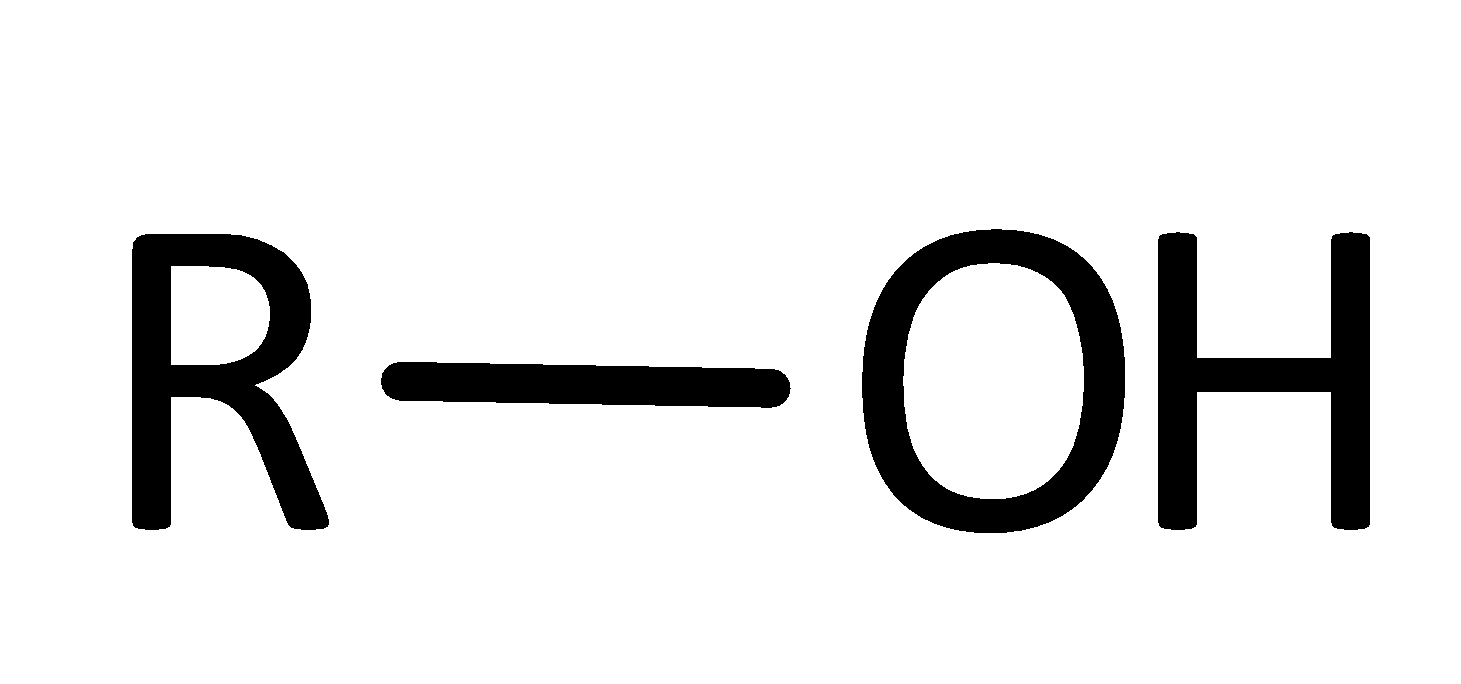
Therefore, option C is correct.
Now, we see details about an aldehyde functional group.
An organic compound that contains a carbonyl group with the central carbon atom bonded to hydrogen and alkyl group is called an Aldehyde group.
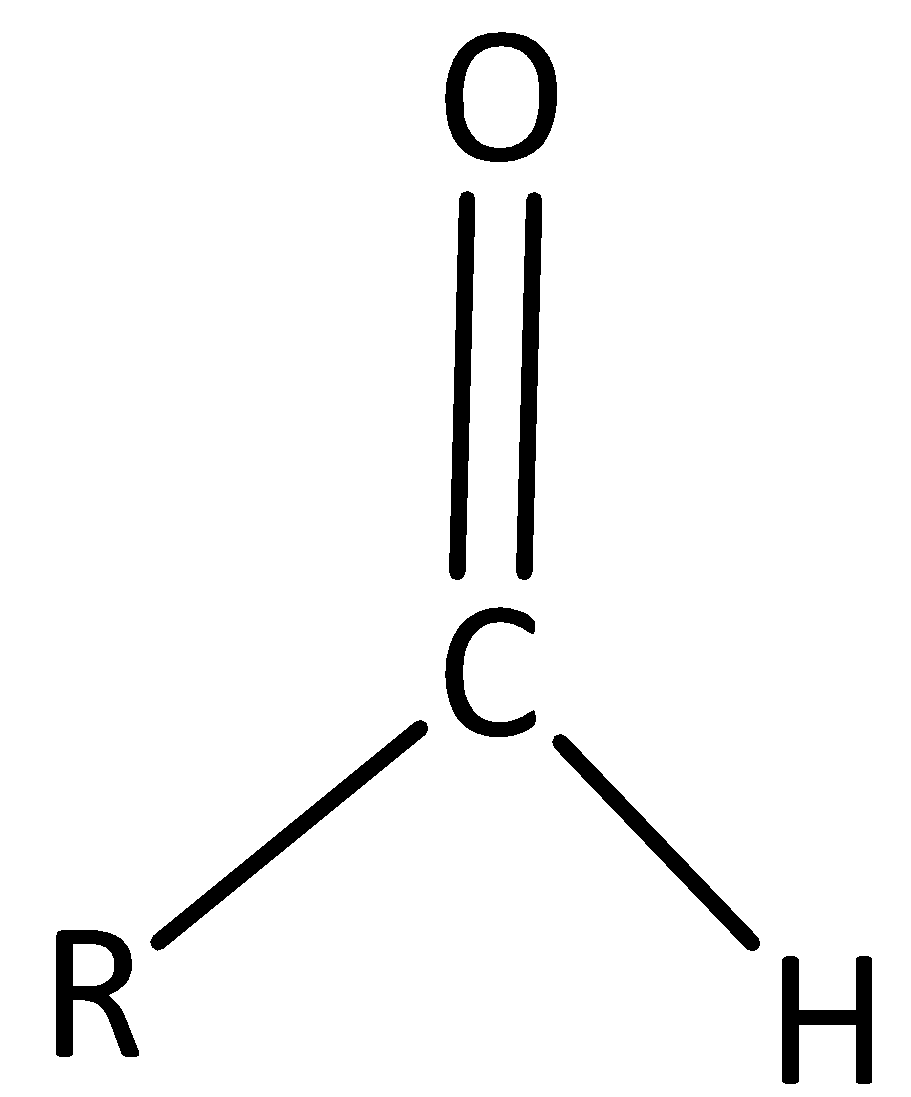
Therefore, option D is incorrect.
The functional group for the acidic group is−COOH.
Therefore, option B is incorrect.
The structure of ester,
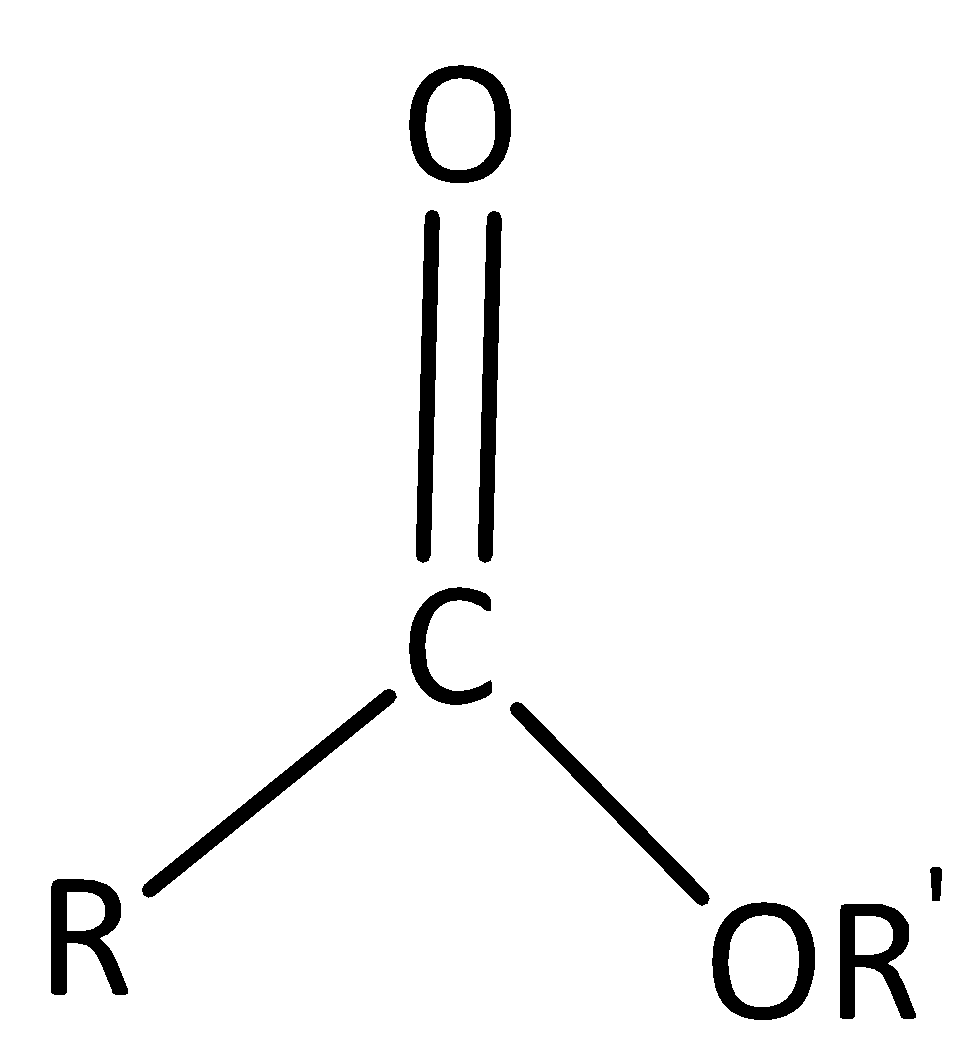
An ester is identified by the orientation and bonding of the atoms, where R and R’ are both carbon-initiated chains of varying length, also known as alkyl groups.
Therefore, option A is incorrect.
So, the correct answer is “Option C”.
Note: We also remember that an organic compound may contain more than one functional group. To identify the functional group we must know their formula.
Example: The formula for the hydroxyl functional group is−OH and the functional group for the acidic group is−COOH.
