Question
Question: The function of \[ZnC{{l}_{2}}\] in Lucas test for alcohols is: a.) To act as an acid catalyst and...
The function of ZnCl2 in Lucas test for alcohols is:
a.) To act as an acid catalyst and react with HCl to form H2ZnCl4
b.) To act as a base catalyst and react with NaOH to form Na2Zn(OH)4
c.) To act as an amphoteric catalyst
d.) To act as a neutral catalyst
Solution
Lucas reagent is a mixture of HCl and ZnCl2. Lucas reagent is used to test the presence of alcohol in the given compound. Alcohols in organic compounds react with Lucas reagent and form Alkyl halides as the products.
Complete step by step answer:
ZnCl2is a Lewis acid because of the presence of empty d-orbitals on Zinc.
Oxygen in –OH group of alcohol forms a coordinate covalent bond with zinc and oxygen acquires a positive charge and Zinc acquires a negative charge.
Therefore oxygen becomes a good leaving group and then the reaction moves towards the formation of the product.
The formed carbonium ion reacts with chlorine in hydrochloric acid and forms alkyl halide as a product.
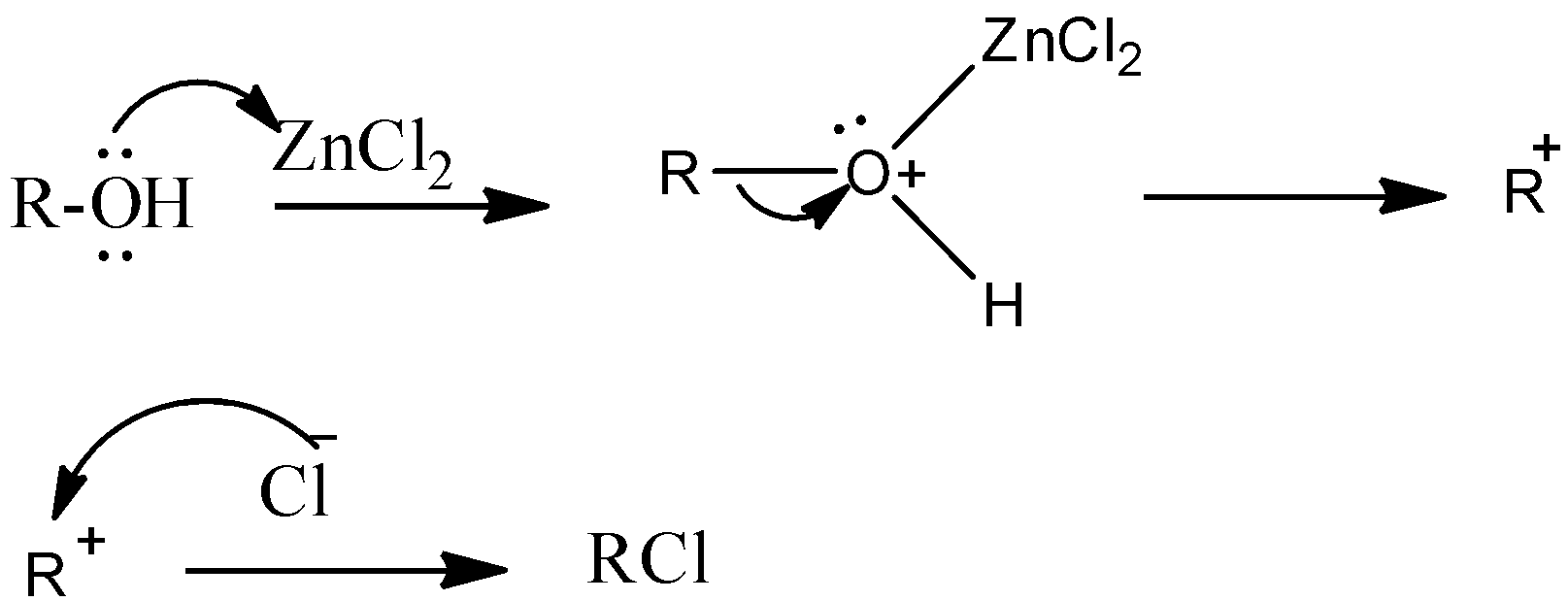
The above reaction can be represented as follows.
R−OH+HClZnCl2R−Cl+H2O
In the above reaction ZnCl2acts as a catalyst.
ZnCl2 reacts with the excess of HCl and forms the following complex.
ZnCl2+2HCl→H2ZnCl4
Therefore ZnCl2acts a catalyst and forms H2ZnCl4by reacting with hydrochloric acid (HCl).
So, the correct answer is “Option A”.
Note: When primary alcohols react with Lucas reagent the solution remains colorless unless it is heated.
Secondary alcohols react with Lucas reagent and form an oily layer in 3-5 minutes.
Tertiary alcohols turn to turbid and form an oily layer immediately by the addition of Lucas reagent.
