Question
Question: The frequency of the characteristic X ray of \({{K}_{a}}\) line of metal target ‘M’ is \(2500c{{m}^{...
The frequency of the characteristic X ray of Ka line of metal target ‘M’ is 2500cm−1 and the graph between √v vs ‘z’ is as follows, then the atomic number of M is?
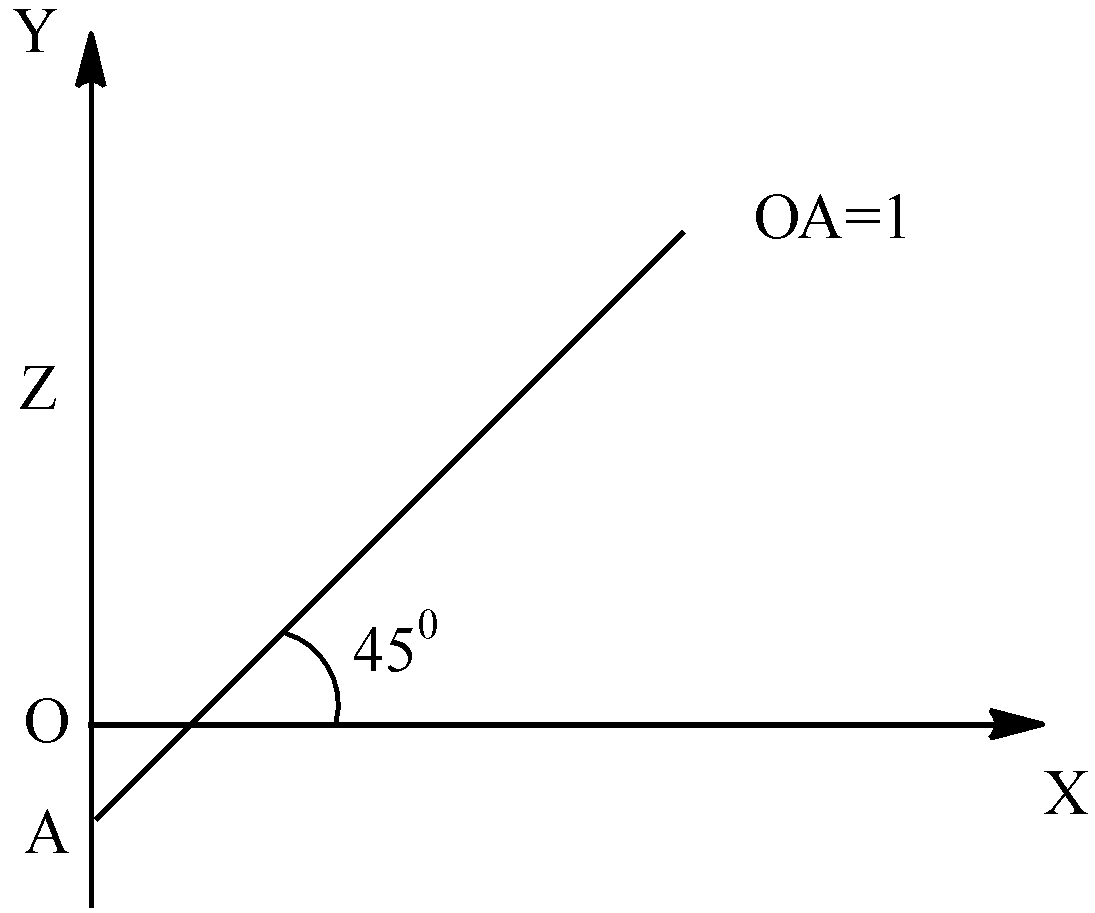
Solution
The concept of Moseley’s Law is to be used in this question. Form an equation containing all the parameters of the Moseley’s Law and compare it with the equation of a straight line and find the unknown constants.
Complete step by step answer:
In order to answer our question, we need to know about the structure of atoms as well as the application of Moseley’s law. Moseley’s law tells us the characteristics when X rays get emitted out by the atoms. The law was introduced by Henry Moseley and the law states that the atomic number is directly proportional to the square root of the frequency of the emitted X ray. Mathematically, vαZ.
- It was found out that the Ka lines had a relation with the atomic number. When it got incorporated with Bohr’s model, the new formula that started Moseley’s Law was:
υ=A(Z−b)2
- Here, υ is the frequency of the X-ray emission line and A and b are the constants which depend on the line type, like K, L, etc.
- Now, let us come to the solution. We have the graph and the frequency of the X Ray as 2500cm−1. Now, according to the Moseley’s Law, we can write:
