Question
Question: The formula used to calculate molar conductivity of an electrolyte is _______....
The formula used to calculate molar conductivity of an electrolyte is _______.
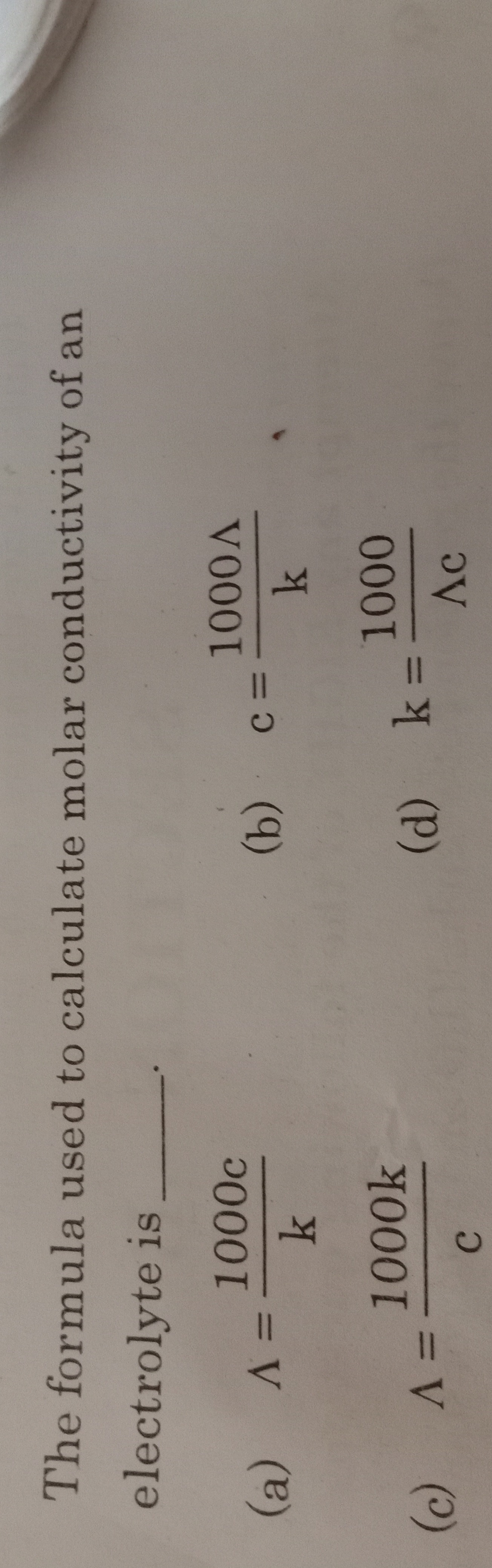
A
Λ=k1000c
B
c=k1000Λ
C
Λ=c1000k
D
k=Λc1000
Answer
Λ=c1000k
Explanation
Solution
Molar conductivity (Λ) is the conductivity of the volume of solution containing one mole of electrolyte. If k is specific conductivity (S cm⁻¹) and c is molar concentration (mol L⁻¹), then the volume containing one mole of electrolyte is 1000/c cm³. Therefore, Λ=k×(1000/c)=1000k/c.
