Question
Question: The formula of one molecule of phosphorus is: \[A.\;\;\;\;\;P \\\ B.\;\;\;\;\;{P_2} \\\ C....
The formula of one molecule of phosphorus is:
B.\;\;\;\;\;{P_2} \\\ C.\;\;\;\;\;{P_4} \\\ D.\;\;\;\;\;{P_8} $$Solution
Hint : Phosphorus forms only three bonds. It exists as tetra-atomic. The size of phosphorus is very large because of which the p-orbitals which usually overlap each other are unable to overlap in phosphorus to make a pie bond. But phosphorus can show catenation.
Complete Step By Step Answer:
The total number of electrons in phosphorus is15. The outer orbital consists of 5electrons in phosphorus, and there are 10electrons in the inner orbitals so the difference between the nucleus and the outer 5electrons is big. So the outer 5electrons are unable to form strong triple bonds. The outer orbital of phosphorus contains a large space because the electrons are far apart from each other and they need to complete their octet state so to become stable and complete its octet phosphorus gets bonded to three other atoms of phosphorus. And so phosphorus becomes tetra atomic which means its atomicity is4. Hence phosphorus exists asP4. Therefore the formula of one molecule of phosphorus is P4
P4 is also known as white phosphorus. The single bonds between the atoms are very weak; this makes phosphorus a very reactive molecule. It is waxy solid and very poisonous. P4 needs to be stored underwater because in the presence of air it burns vigorously. When it burns in the air and gives diphosphorus pentoxide. It smells like garlic. In both solid and vapor states the phosphorus molecule exists asP4. The structure of P4is shown below:
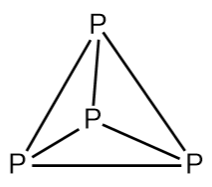
Hence from the above information, we can say that formula of one molecule of phosphorus is P4
Therefore the correct option is C.P4.
Note :
Phosphorus has three allotropes white phosphorus, red phosphorus, and black phosphorus. Both white and red phosphorus exist as P4 molecules whereas black phosphorus exists in two different forms α−black−P andβ−black−P. Among the three white phosphorus is highly reactive.
