Question
Question: The following structure represents cyclopentyl methyl carbinol. State whether the given statement ...
The following structure represents cyclopentyl methyl carbinol.
State whether the given statement is true or false:
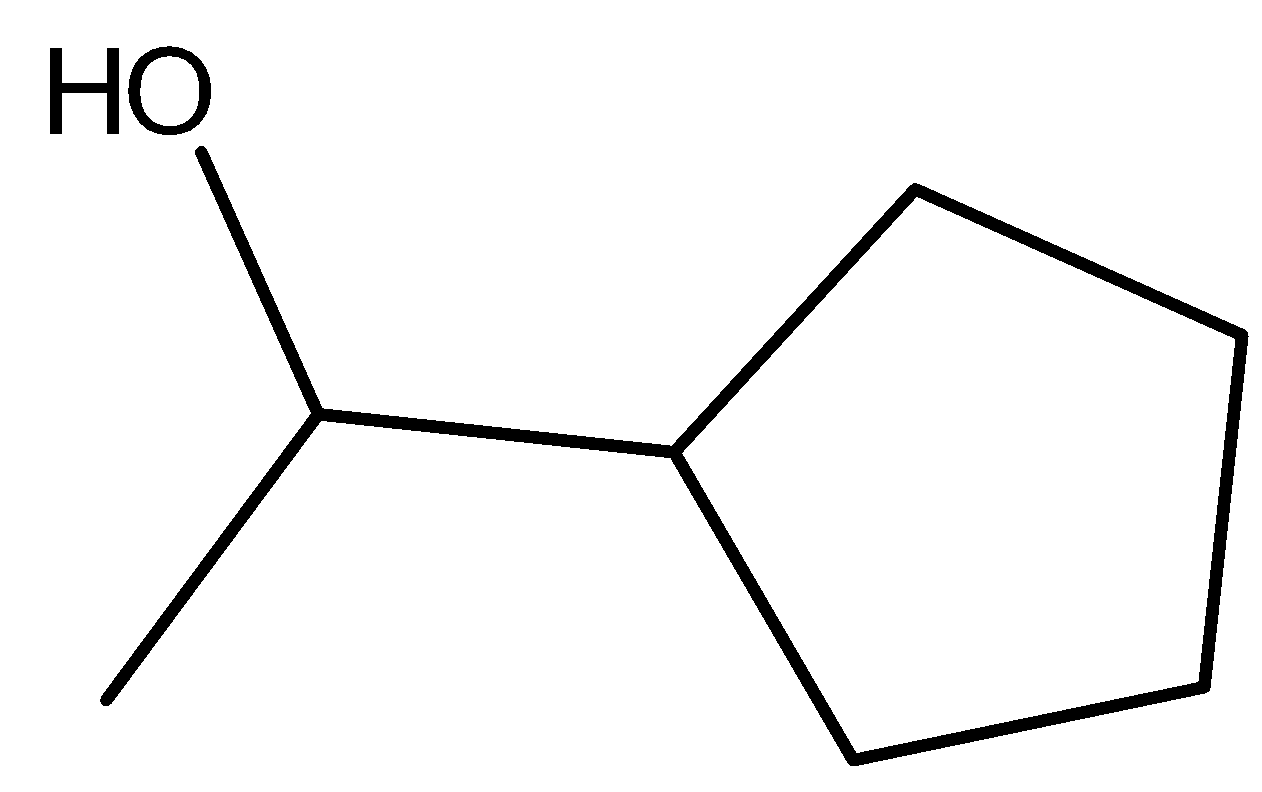
A) True
B) False
Solution
Follow the names given in the question and find out the required structures for that name in the given structure. The CH3OH group is also commonly called as the carbinol. To solve this question one needs to find out whether that group is present in the structure or not.
Complete step by step answer:
- The given name is as per the common name nomenclature system which we will analyze by doing fragments of the structure step by step as follows.
- The word cyclopentyl stands for a cyclic ring which has five carbon atoms in it without having any double bonds in the ring. We have identified a cyclic ring in the structure which stands for cyclopentyl structure.
- The next word methyl stands for the group −CH3 which is present in the structure at the terminal end.
- The next word carbinol represents the chemical structure −CH3−OH and by analyzing the structure, there is a presence of carbinol in the structure. The carbinol group in the structure has been shown in the following representation:
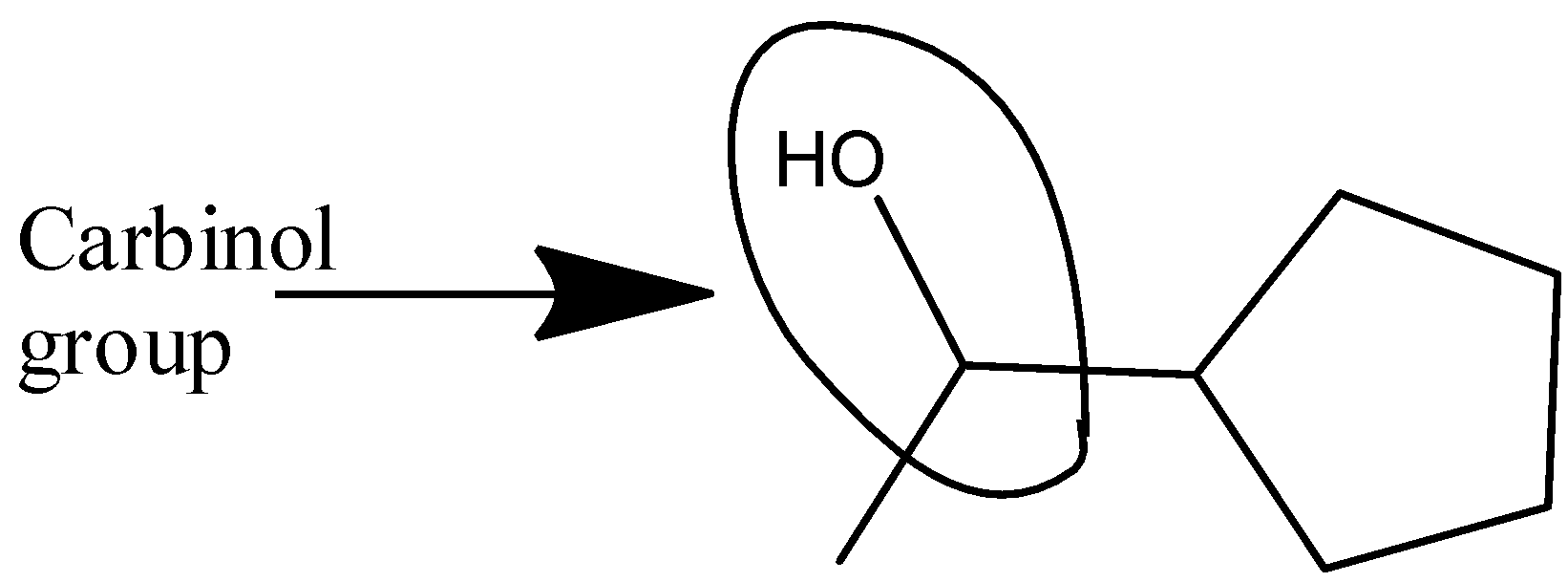
- Therefore, as per our step by step analysis of the structure, we have come to a conclusion that all the names in the structure are present in the chemical diagram given in the question.
- Hence, the given statement is true which shows that option A is correct.
Additional information: According to the IUPAC nomenclature system, the IUPAC name of the structure is 1−cyclopentylethanol. The carbinol group is commonly known as the methyl alcohol group. For the chemical identification of the carbinol group in the structure, the test used for the identification of alcohol can be used such as sodium metal test, iodoform test, etc.
Note:
The carbinol group stands for −CH3OH. The carbinol name is used commonly in the Common name nomenclature system. The presence of the methanol group anywhere in the structure will show the presence of the carbinol group.
