Question
Question: The following reaction is used for the preparation of oxygen gas in the laboratory. \( 2KCl{{O}_...
The following reaction is used for the preparation of oxygen gas in the laboratory.
2KClO3HeatCatalyst2KCl+3O2
Which of the following statements is correct about the reaction?
(A) It is a decomposition reaction and endothermic in nature
(B) It is a combination reaction
(C) It is a decomposition reaction and accompanied by release of heat
(D) It is a photochemical decomposition reaction and exothermic in nature
Solution
Hint : Here, the reaction for production of oxygen using potassium chlorate in the laboratory is given. Potassium chlorate contains three oxygen bonds with chlorine with covalent bonds and the whole molecule makes ionic bonds with potassium. As we can see from the given reaction, the bonds will be broken, and we know that energy or heat is required to be provided from a source to break the bonds in a molecule.
Complete Step By Step Answer:
The IUPAC name of the given compound KClO3 is Potassium Chlorate. Let us draw the structure of Potassium chlorate for better understanding as shown.
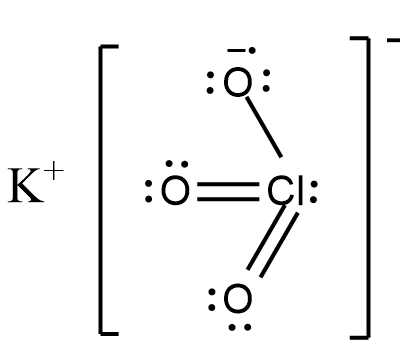
Two out of three oxygen connected to chlorine are connected by a simple double bond. The third oxygen is bonded with a single bond and it accepts an electron from potassium due to higher electronegativity and hence, forms an ionic bond with potassium.
Now, due to the structure and number of bonds, the molecule resonates in which the double bond resonates between the single bond and double bond. Due to bond resonance, the electron also does not remain local.
Due to these factors, the chlorate ion becomes very strong and stable and heat or energy and catalyst is required to break the bond and carry out the reaction.
Hence, as we are giving heat to the reaction from an outside source, the heat will be added to the system and the reaction proves to be endothermic.
A decomposition reaction is a reaction in which a bigger molecule breaks into simpler smaller molecules.
Here, potassium chlorate breaks down to simpler molecules like Potassium Chloride and Water.
Hence, the given reaction is decomposition reaction
Hence, the correct answer is Option (A) .
Note :
Here, in the reaction, olds bonds are broken for which energy or heat will be required and new bonds are formed for which energy or heat is released. But, the chlorate ion is a resonating molecule and hence, high energy is required to break the bonds. Hence, even though energy will be released, the energy requirement will be much higher and thus, the reaction will be endothermic.
