Question
Question: The following question represents the variation of boiling point with the composition of liquid and ...
The following question represents the variation of boiling point with the composition of liquid and vapours of binary liquid mixtures. The graph is plotted at constant pressure. Which of the following statement(s) is incorrect? Here X & Y stands for mole fraction in liquid and vapour phase respectively
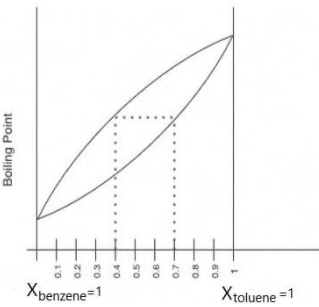
A) Xbenzene=0.5andYtoluene=0.2
B) Xtoluene=0.3andYbenzene=0.6
C) Xbenzene=0.3andYtoluene=0.4
D) ifXbenzene=0.7thanYtoluene<0.3
Solution
We can use Raoult’s law to solve this question, which states that in presence of non-volatile solute reduces the vapour pressure of the solution in comparison to vapour pressure of pure solvent by occupying the position of solvent molecules on the liquid surfaces. Thus, it reduces the escaping tendency of solvent molecules into the vapour phase.
Complete step-by-step answer:
We can see in the graph that it is plotted against boiling point and the mole fraction of the solution.
From the definition of boiling point, we understood that it is the temperature at which vapour pressure of liquid becomes equivalent to the pressure of its atmosphere.
So, when the mole fraction of benzene is 1, i.e.,Xbenzene=1, its pressure will be the pressure of its pure solute. And when the mole fraction of toluene is 1 i.e.,Xtoluene=1 its pressure will be of its pure solute.
Also by the definition of boiling point and observing the graph, we understood that,
The boiling point of pure benzene is less than that of the boiling point of toluene
Which can be concluded as that,
Ptoluene∘<Pbenzene∘
Therefore, we can say that benzene is more volatile than toluene.
From Raoult’s law, we know that
Psolute=Psolute∘χSolute
From this, we get a clear idea that the above one is the vapour state and the below one is in a liquid state.
So, we can say from the graph that
Xbenzene<Ybenzene
And,
Xtoluene<Ytoluene
Now coming to our options we can say that option (A), (B) and (D) are clearly correct. Only option (C) is incorrect statement
Therefore, option ‘C’ is the correct answer.
Note: Remember that more volatile liquid can be purified from the mixture by collecting and recollecting and this process is commonly known as distillation. Also, remember that Raoult’s law gives the relationship between vapour pressure and concentration of the solution.
