Question
Question: The following graph shows two isotherms for a fixed mass of an ideal gas. The ratio of r.m.s speed o...
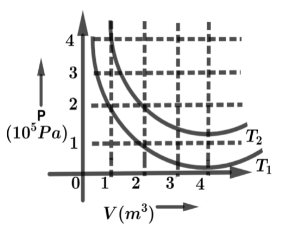
The following graph shows two isotherms for a fixed mass of an ideal gas. The ratio of r.m.s speed of the molecules at temperature T1 and T2 is:
Solution
In the question there given a graph showing the two isotherms for fixed ideal gas, from the given graph we have to find the root mean square of the ratio of T1 and T2. For this we are using the rms formula for calculating the ratio of speed of molecules.
Complete step by step answer:
Given temperature T1 we are calculating the rms value,
Then V1=M3RT1→(1)
For temperature T2 the rms value is,
V2=M3RT2→(2)
By diving the rms value equation (1) divides (2),
V2V1=T2T1→(A)
We Have find the rms value for two temperatures,
Now from the graph, the PV are constant in both the temperature curves in graph
So from graph PV=C constant in two temperatures
From graph the temperature T1 written as, 2×1=nRT1
From graph in temperature T1 the curve meets the points (2,1) so that we have written as,
2×1=nRT1→(3)
Same as for temperature T2 curve, where it meets the points in (2,2), so we have written for second temperature T2 is
2×2=nRT2→(4)
Now we are going to divide the equations (3) and (4),
Therefore,
42=nRT2nRT1 ⇒T2T1=21
Now we are substituting the values of ratio of two temperatures in the rms ratio, then we get
V2V1=21
We can also write the above equation in,
∴V2V1=21
Thus we have proved the ratio of rms speed of the molecules for two temperatures.
Note: From the given graph only we are proved the ratio of rms speed of the molecules of the temperatures T1 and T2. To find this ratio first of all we have proven the rms of temperature and then we find the temperature from points taken from X-axis and Y-axis for two temperatures. Then we find the ratio of rms speed of the molecules.
